Example Problem - Pyrolysis of Benzene
Aspen Plus will be introduced with benzene pyrolysis reaction in a plug flow reactor in this tutorial. The next three pages will present the details and the data.
Diphenyl (C12H10) is an important industrial intermediate. One production scheme involves the pyrolytic dehydrogenetation of benzene (C6H6) [1]. During the process, triphenyl (C18H14) is also formed by a secondary reaction.
The reactions are as follows:
(1)![]()
(2)![]()
Substituting the symbolic IDs A = C6H6, B = C12H10, C = C18H14 and D = H2
(3)![]()
(4)![]()
Murhpy, Lamb and Watson presented some laboratory data regarding these reactions originally carried out by Kassell [2]. In these experiments, liquid benzene was vaporized, heated to the reaction temperature and fed to a plug flow reactor (PFR). The product stream is condensed and analyzed for various components. The results are tabulated in Table 1.
Table 2 Laboratory data for P = 1 atm.
|
Temperature (°F) |
Flow rate (lbmole/hr) |
yA |
yB |
yC |
yD |
|
1400 |
0.0682 |
0.8410 |
0.0695 |
0.00680 |
0.0830 |
|
1265 |
0.0210 |
0.8280 |
0.0737 |
0.00812 |
0.0900 |
|
1265 |
0.0105 |
0.7040 |
0.1130 |
0.02297 |
0.1590 |
|
1265 |
0.0070 |
0.6220 |
0.1322 |
0.03815 |
0.2085 |
|
1265 |
0.0053 |
0.5650 |
0.1400 |
0.05190 |
0.2440 |
|
1265 |
0.0035 |
0.4990 |
0.1468 |
0.06910 |
0.2847 |
|
1265 |
0.0030 |
0.4820 |
0.1477 |
0.07400 |
0.2960 |
|
1265 |
0.0026 |
0.4700 |
0.1477 |
0.07810 |
0.3040 |
|
1265 |
0.0007 |
0.4430 |
0.1476 |
0.08700 |
0.3220 |
|
1265 |
0.0003 |
0.4430 |
0.1476 |
0.08700 |
0.3220 |
Additional data
A = C6H6 B = C12H10 C= C18H14 D = H2
The reactor tube dimensions:
L = 37.5 in, D = 0.5 in
Rate laws
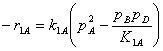
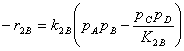
Specific reaction rate constants
![]()
![]()
Equilibrium constants
![]()
![]()
Parameter values
E1 = 30190 cal/mol A1= 7.4652E6 lbmole/h/ft3/atm2
E2 = 30190 cal/mol A2= 8.6630E6 lbmole/h/ft3/atm2
A' = -19.76 A''=-28.74
B' = -1692 B''=742
C' = 3.13 C''= 4.32
D' = -1.63E-3 D''=-3.15E-3
E' = 1.96E-7 E'' = 5.08E-7
P = 14.69595 psi R = 1.987 cal/mol/K
Exercise
Follow the instructions during the lab session and use the handouts to replicate the data presented in Table 1 for T = 1400 °F and P = 1 atm using Aspen Plus™. What is the percent difference between experimental and simulated mole fractions?
References
[1]H.S. Fogler, Elements of Chemical Reaction Engineering, 3rd ed., p.77-79, Prentice Hall, New Jersey, 1999.
[2]G.B. Murphy, G.G. Lamb, and K.M. Watson, Trans. Am. Inst. Chem. Engrs., (34) 429, 1938.