Reactions input forms
In these forms we will first enter the stoichiometric and power law coefficients for all components in each reaction, and then we will move on to kinetics tab. In Aspen Plus™ notation we will represent the two reversible reactions as four separate reactions, each with their own kinetic expression. Select New to proceed and fill the boxes as suggested by the following pictures.
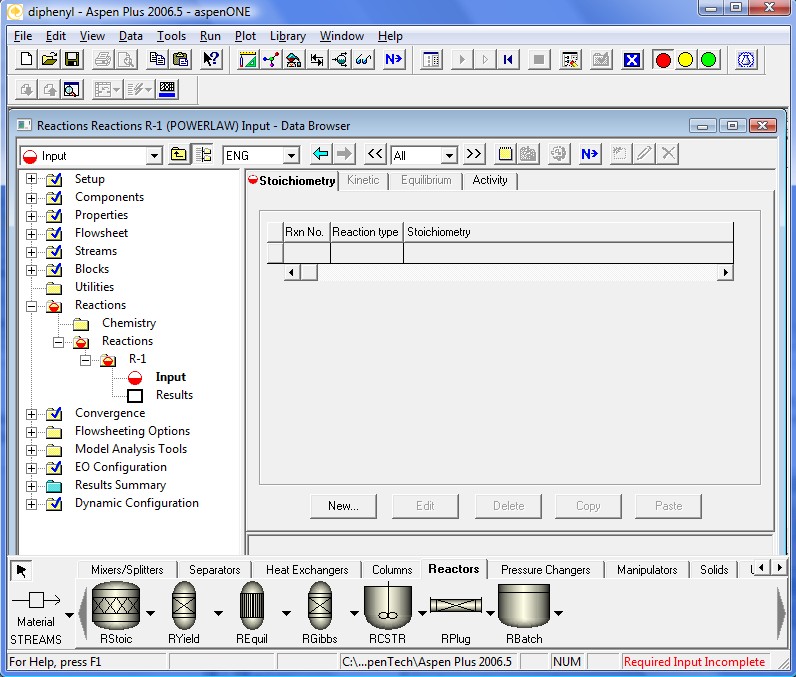
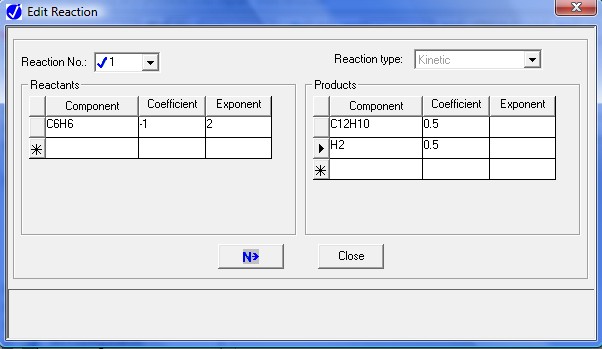
After completing the first reaction select New from Reaction No. list. Next picture will show the coefficients for the reversed part of the first reaction. In Aspen Plus™ notation, this will be our 2nd reaction
In these entries note that we brought all species on a "per mole of benzene" basis. This is an important detail.
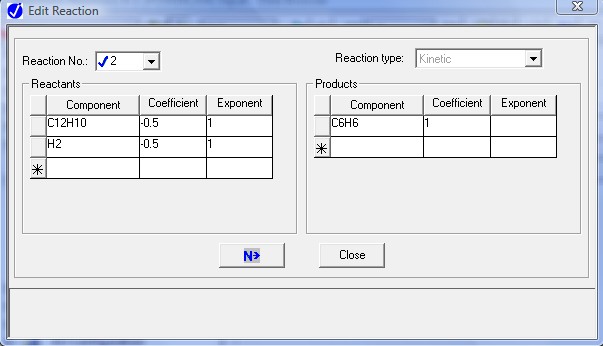
Similarly, enter the last two equations. The next screen will show the resulting stoichiometric relations.
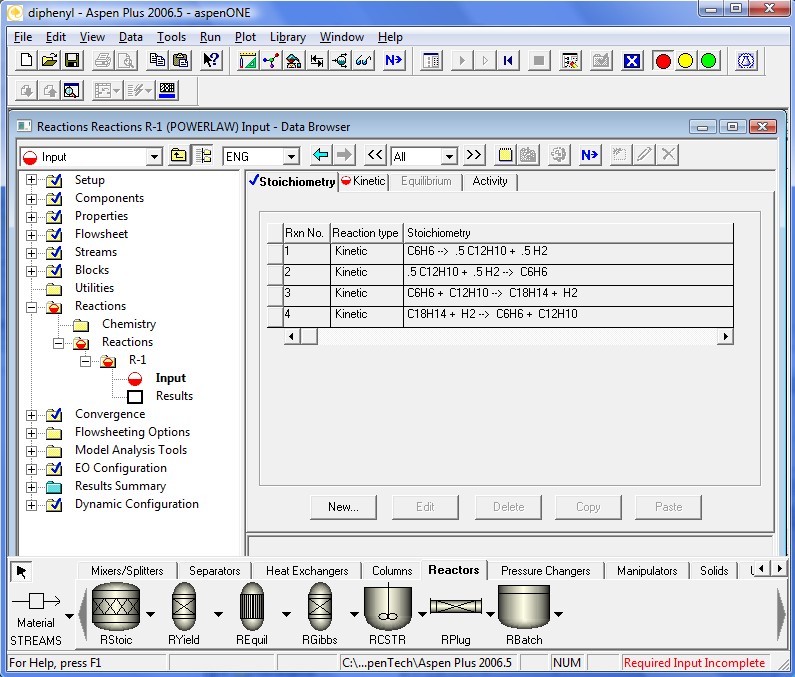
The kinetics coefficients are specified in the following four pages. Note that the definition of the kinetic factor expression is a little different. It is a more general definition for the specific reaction rate constant. When To is ignored Aspen Plus™ defaults back to simpler Arrhenius Equation. Also note that k should be specified in SI units regardless of the units used elsewhere.
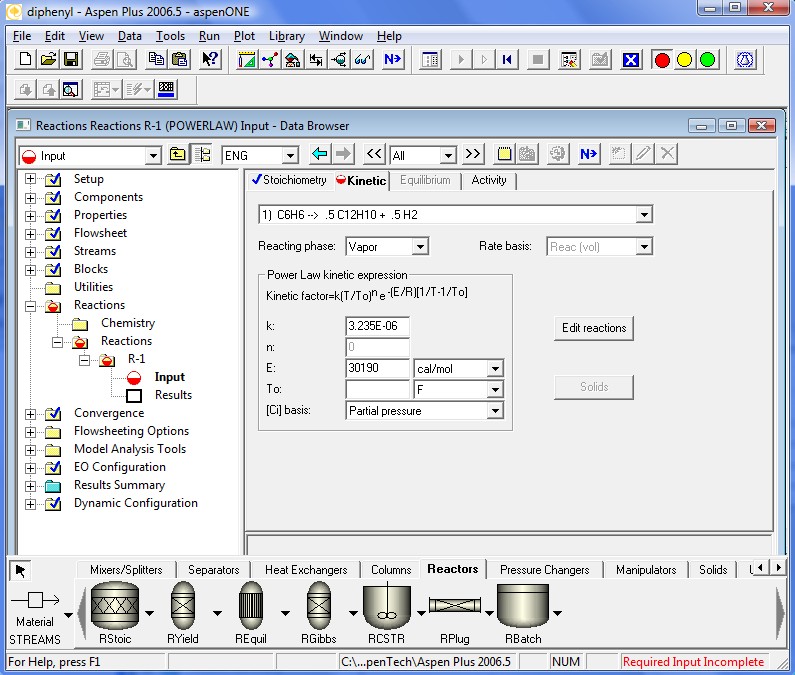
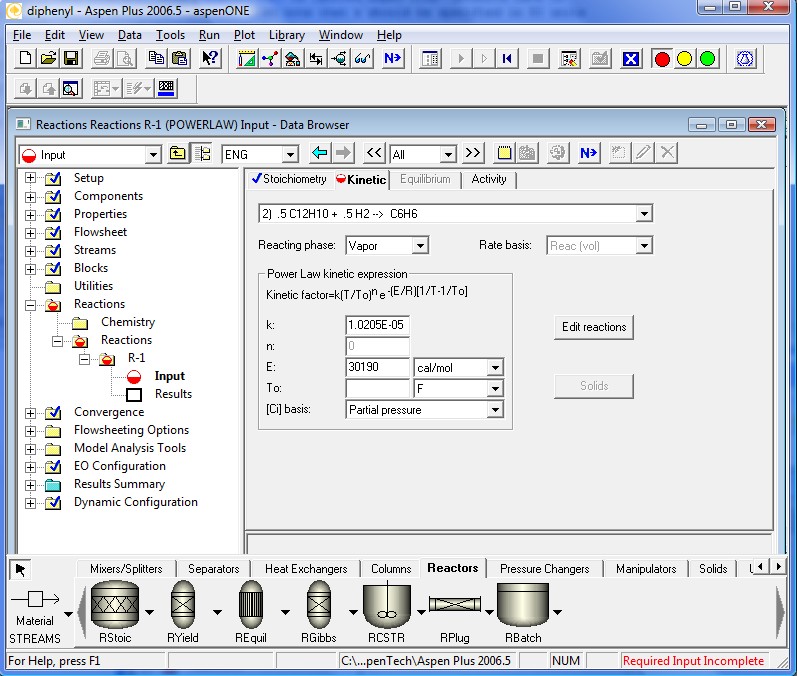
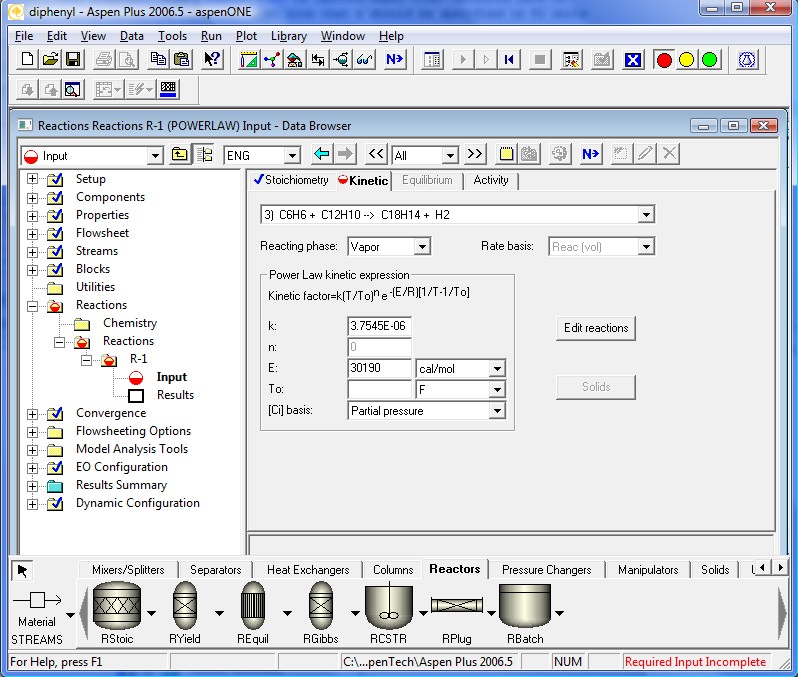
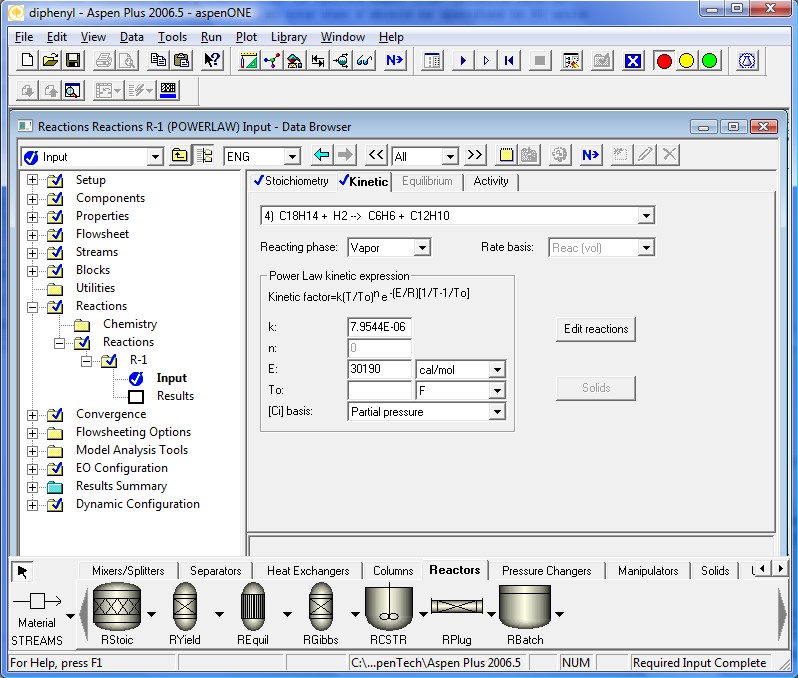
All required input is now complete. We are ready to run the simulation. Clicking the Next Button will invoke the following dialog.
