MECHANISM OF B12 HOMOLYSIS
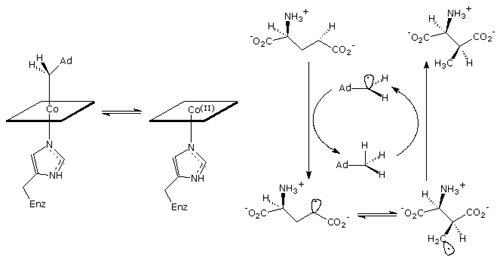
The first step in all adenosylcobalamin (AdoCbl) mediated reations is homolysis of the cobalt-carbon bond which serves to "unmask" the catalytic 5'-deoxyadenosyl radical. This then reacts with the substrate to remove a hydrogen atom and initiate the rearrangement as shown in the above scheme.
In free solution the cobalt-carbon bond-dissociation energy is 30 kcal/mol, but to achieve the rates of catalysis seen in the AdoCbl-dependent isomerases, this bond is thought to be substantially weakened when the enzyme binds the coenzyme. We have used stopped-flow spectroscopy to measure the rates of AdoCbl homolysis when the enzyme is reacted with glutamate and methylaspartate. Either substrate elicits very rapid cleavage of the coenzyme: the rate accceleration is estimated as at least 1012-fold over the uncatalysed reaction.
Unexpectedly we observed very large deuterium isotope effects of ~30-fold,
when the enzyme was reacted with substrates containing deuterium in the
abstractable position. This coupling of homolysis and hydrogen abstraction
could arise if hydrogen transfer and cobalt-carbon bond homolysis occur
simultaneously in a concerted fashion. Alternatively, adenosyl radical
could exist at very low concentration, in a rapid but unfavorable equilibrium
with AdoCbl, that is displaced towards homolysis by the subsequent reaction
of adenosyl radical with substrate in a slow step.
In either case it appears that the 5'-deoxyadenosyl radical, a species that is central to mechanistic thinking regarding the role of AdoCbl, can only exist at very low concentrations in the enzyme, and indeed may not even be a true intermediate in these reactions. This may explain why this species has never been observed spectroscopically. the magnitude of the isotope effects observed in these experiments raises the possibility that quantum tunneling may be important in the transfer of hydrogen between substrate and coenzyme. This work was recently published in Biochemistry.