HOW CHROMATOGRAPHY
WORKS
Factors that affect the shape of chromatograms.
What is a good chromatograph?...
In order to obtain accurate
measurements, it is necessary to have an understanding of what
factors in our chromatography experiments affect the shape of
chromatograms and thus our ability to characterize and separate
mixtures. This is where science becomes art, as continued lab
experience and practice with chromatography is how best to learn to
finesse results into accuracy. However, we will discuss two main
topics, resolution and band
broadening, and how flow rate and stationary/mobile phase
interaction can
affect them and our ability to obtain a good chromatogram.
Resolution
Resolution is a measure of our ability
to distinguish between two different peaks. If two eluents have
similar retention times, then their chromatograms may blend together
and appear as one curve. Thus we can easily lose track of mixture
components and inaccurately compute retention times and
concentrations for false peaks.

The above chromatogram appears to be have only one peak, but we
can detect a slight anomaly -- the notch in the top -- which might
suggest that there is more there than we thought. We require a way
to increase the time between these retention times. Recall that
retention time is a function of the mobile phase-stationary phase
interaction. The nature of our mobile phase and stationary phase and
the equibrium of our analytes between the two phases determines their
retention times. Each chromatography type has its own methods for
adjusting these interactions, such as temperature and concentration
control, that directly affect the quality of the phases
themselves.
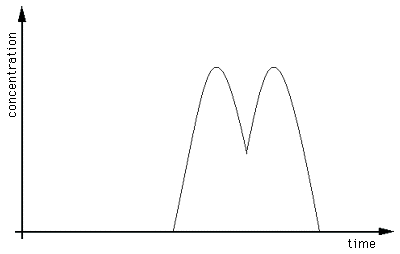
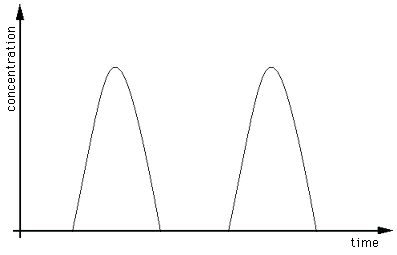
Increased resolution demonstrates the presence
of two analytes.
Band Broadening
While two peaks might have adequately space retention times,
precision problems can arise if the peaks themselves are not sharp enough.
Band broadening is an increase in width in our peaks. If we refer back to
our discussion of Gaussian distributions, recall
that chromatograms are useful because they graphically afford us a mean retention
time. The standard deviation is a graphical measure of the relative width
of these curves and mathematically an indicator of the total data's proximity
to the mean value. We might have an average retention time, but if the standard
deviation is too great, then the mean is not a precise representation of the
entire data collection and therefore is not as useful.
 Band broadening arises from the same mobile/stationary
phase interaction as described above. There is a greater variation
in the type of interactions that analyte molecules are having with
the phases, causing a wider distribution of retention times. Careful
choice of mobile and stationary phases will also help in curtailing
this phenomena. Another possible solution, which could also assist
with the previous resolution problem, would be to alter the flow rate
of the mobile phase. Adjusting the flow rate changes the ability of
the analyte to interact with the stationary phase. Increasing the
carrier flow will cause a faster elution because the analyte will
have less time to spend interacting with the stationary phase and
thus less time IN the stationary phase. Decreasing the carrier flow
allows more time for these interactions to occur and will increase
band broadening while increasing resolution.
Band broadening arises from the same mobile/stationary
phase interaction as described above. There is a greater variation
in the type of interactions that analyte molecules are having with
the phases, causing a wider distribution of retention times. Careful
choice of mobile and stationary phases will also help in curtailing
this phenomena. Another possible solution, which could also assist
with the previous resolution problem, would be to alter the flow rate
of the mobile phase. Adjusting the flow rate changes the ability of
the analyte to interact with the stationary phase. Increasing the
carrier flow will cause a faster elution because the analyte will
have less time to spend interacting with the stationary phase and
thus less time IN the stationary phase. Decreasing the carrier flow
allows more time for these interactions to occur and will increase
band broadening while increasing resolution.
Methods for achieving better separations are
discussed in more depth in our separate treatments of chromatography
types.
BACK...