IR in the Liquid Phase and Neat Samples
 Salt plates are
used to take an IR spectra of a liquid sample. The plates work the
same way as the potassium bromide for solid samples. The major
problem of using a liquid sample is choosing a solvent with which to
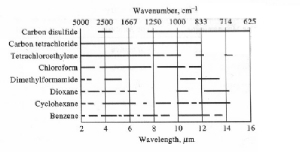
dilute the sample. The list below shows the areas that the solvents
are usable (Carbon disulfide is usable around 2500 cm-1
and then after 1250 cm-1, etc.).
Salt plates are
used to take an IR spectra of a liquid sample. The plates work the
same way as the potassium bromide for solid samples. The major
problem of using a liquid sample is choosing a solvent with which to
dilute the sample. The list below shows the areas that the solvents
are usable (Carbon disulfide is usable around 2500 cm-1
and then after 1250 cm-1, etc.).
No solvent is perfect but if some information about the compound
is known, then a solvent can be chosen accordingly. Notice that water
is not a solvent on this list. First, water
is a bad solvent because it will dissolve the salt plates. Second, water
exibits a broad -OH peak that
will cover up a lot of other peaks that you are interested in. These
two reasons are good enough to NEVER USE
WATER WHEN TAKING AN IR.
Back to Sample Preparation
 Salt plates are
used to take an IR spectra of a liquid sample. The plates work the
same way as the potassium bromide for solid samples. The major
problem of using a liquid sample is choosing a solvent with which to
dilute the sample. The list below shows the areas that the solvents
are usable (Carbon disulfide is usable around 2500 cm-1
and then after 1250 cm-1, etc.).
Salt plates are
used to take an IR spectra of a liquid sample. The plates work the
same way as the potassium bromide for solid samples. The major
problem of using a liquid sample is choosing a solvent with which to
dilute the sample. The list below shows the areas that the solvents
are usable (Carbon disulfide is usable around 2500 cm-1
and then after 1250 cm-1, etc.).