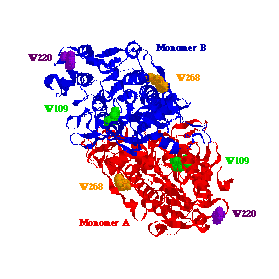
 Figure 5: The X-ray crystal structure of Escherichia coli AP. AP
is a dimeric metalloenzyme with a total molecular weight of about
Figure 5: The X-ray crystal structure of Escherichia coli AP. AP
is a dimeric metalloenzyme with a total molecular weight of about
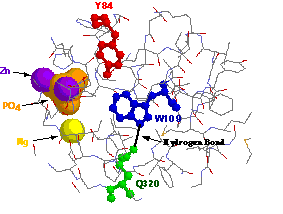 Figure 6: Close-up view of the hydrophobic core of AP around
tryptophan 109. A hydrogen bond is formed between glutamine 320 and
the enamine of tryptophan 109 which stabilizes the indole ring. The
vicinity of tyrosine 84 makes it a candidate for collisional quenching
of tryptophan 109ís phosphorescence.
Figure 6: Close-up view of the hydrophobic core of AP around
tryptophan 109. A hydrogen bond is formed between glutamine 320 and
the enamine of tryptophan 109 which stabilizes the indole ring. The
vicinity of tyrosine 84 makes it a candidate for collisional quenching
of tryptophan 109ís phosphorescence.
Also shown is the close proximity of the phosphate and metal binding sites.