Activation Energies For Hydrogen Exchange
Arrhenius plots of the observed H/D exchange rates gives us the activation energy for the exchange process. We expect that removing the hydrogen bond to the enamine will speed the exchange for that protein and that is indeed what we observe [Figure 6, below]. Although the reaction rates are faster for both mutants, they are orders of magnitude faster for Q320L. In addition the activation energy for the exchange is reduced for Q320L but relatively unaffected for Q320G. We are currently making crystals of these mutants to learn more about how the mutations have affected the protein's structure.
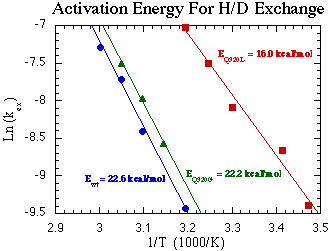
Figure 6: Calculated activation energies for the H/D exchange process for WT, Q320L and Q320G AP.
Conclusions