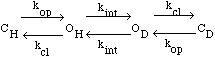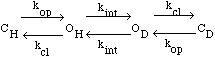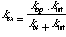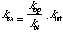Hydrogen Exchange Theory
The accepted model of protein hydrogen exchange as a two-step process was
described in detail by Hvidt and Nielsen
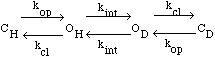
In this model CH and CD represent the protonated and
deuterated closed
(unable to exchange) forms of the residue. OH is the
protonated open,
exchangeable form. kop is the rate constant for the opening
step and kcl
is the rate constant for the closing step. kint is the rate
constant for
the exchange reaction of the exposed hydrogen. The observed rate constant
for the exchange process, kex, is given by
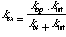
There are two limiting cases for this model. If kcl >
kint then
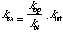
and the process is said to follow EX2 kinetics. In the other limit,
termed EX1, kcl < kint and the rate limiting step
in the process is the
opening step since kex=kop. Protons that exchange
following EX2 kinetics
are usually solvent exposed in the native state of the protein or require
only smaller structural fluctuations to expose them. In contrast, protons
exchanging with EX1 kinetics are generally deeply buried and require a
more global fluctuation in the structure of the protein to be exposed to
the solvent. Determining which limit best describes the exchange of a
particular proton can be a powerful tool for determining the relative
location of that proton within the protein; i.e., how deeply buried or
protected is it from the solvent.
Experimental
Results