P.F.S.R.L. - RTP Background

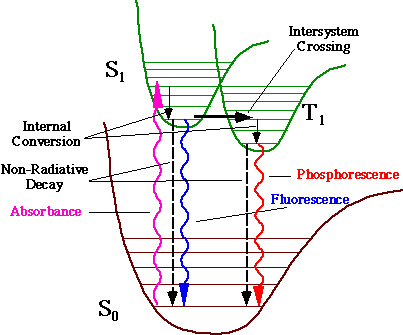 |
Two types of luminescence observed from proteins are fluorescence and
phosphorescence. Fluorescence arises from the decay of the
excited single state S1
to the ground state S0. Phosphorescence
arises from the decay of the excited triplet state T1 to the ground state S0. Phosphorescence is strictly
forbidden by quantum mechanics since it involves
a transition between pure states of different spin multiplicity, however,
this forbiddenness can be relaxed by interactions
between the magnetic dipoles generated by the spin of the electron and the
orbital motion of the electron. These spin-orbit interactions lead to
coupling of the singlet and triplet states, giving some singlet character
to the triplet state, and thus to a small, but finite, transition
probability, between T1 and S0.
|
Spin-orbit coupling is weak in planar, aromatic hydrocarbons like
tryptophan so other perturbations must also be included to explain the
long phosphorescence lifetime observed. The important perturbation is
spin-vibronic coupling which couples together nuclear vibrational motions
to the electronic states giving rise to further mixing of singlet and
triplet states.
Molecular oxygen is an excellent quencher of phosphorescence and so room
temperature phosphorescence (RTP) from tryptophans in proteins was not
discovered until the samples were thoroughly deoxygenated2.
(Proteins were observed to phosphoresce at only low temperature before
this time). Since that discovery was made, rtp has been detected
in many proteins1,3,4
and has been shown to be a useful tool for studying protein dynamics and
stability.

Main Page
Current Research
AP Research



![]()