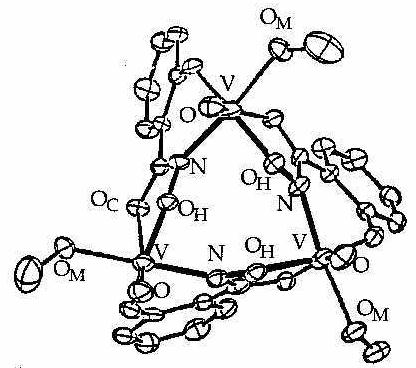
Figure 1 ORTEP diagram for [9-MC(V(V)O)N(shi)-3]. The first Metallacrown (no cavity metal).

Figure 1 ORTEP diagram for [9-MC(V(V)O)N(shi)-3]. The first Metallacrown (no cavity metal).
The first MC to encapsulate a transition metal was Fe(III)(OAc)3[9-MCFe(III)N(Shi)-3] shown in figure 2 below. The MC formed by adding equimolar amounts of Fe(II) sulfate to H3shi in methanol in the presence of three equivalents of sodium acetate to fully deprotonate the ligand. Air oxidizes Fe(II) to Fe(III). The acetate anions neutralized the triply positive charge on the captive iron, and formed bridges between this iron and those of the metallacrown ring. By the formation of this metallacrown and the previous one, two characteristics were enhanced in the crown ether: first was the binding affinity of the oxygens, now negatively charged instead of neutral and better at binding the cation in the cavity, and second was the stability of the crown, with anions bridging the ring metals and the transition metals of the cavity.
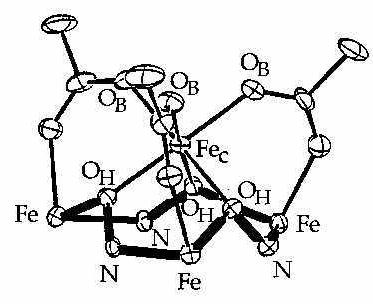
Figure 2 ORTEP diagram for Fe(III)(OAc)3[9-MCFe(III)N(shi)-3]. The first transition metal binding metallacrown.
An inverse 9-MC-3 was synthesized. The positively charged copper, in the metallacrown is oriented towards the center instead of the electronegative oxygen atoms, and anions are selected for as a result. In this case, the cavity captivates hydroxides, while m3-tripodal sulfate binds to the three ring coppers(II).