
Introduction
Vanadium has been found to play a number of roles in biological
systems.
It is present in certain vanadium dependent haloperoxidase and nitrogenase
enzymes. Many sea squirts accumulate vanadium in very high concentration,
although the reason is not known. The Amanita muscaria mushrooms
also accumulate vanadium in the form of a coordination complex called
amavadin, whose function is also unknown. Finally, a number of vanadium
complexes have been shown to alleviate many of the symptoms of diabetes in
both in vitro and in vivo (in rats and mice) studies. These
complexes are being studied as potential alternatives to insulin
therapy.
Research in the Pecoraro group has concentrated primarily on
designing spectroscopic and functional models for vanadium dependent
haloperoxidases (VHPOs). VHPOs catalyze the 2-electron oxidation of a
halide (X- = Cl-, Br-, or I-)
by peroxide through a Lewis acid-promoted mechanism (as opposed to redox
cycling at the vanadium center) as summarized in Scheme 1. A reactive
halogenating species (HOX, X2, or X3-)
is produced which can react with electron-rich substrates to form
halogenated organic compounds. Vanadium remains in the +5 oxidation state
throughout the entire catalytic cycle. When the vanadium is reduced to
the +4 oxidation state, the enzyme is totally inactive.
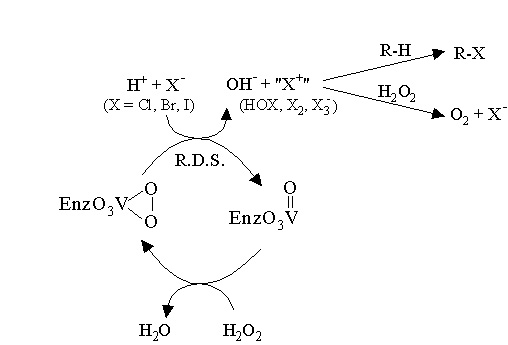
Scheme 1. Proposed mechanism for VHPOs: Vanadium
activates hydrogen peroxide towards nucleophilic halide
attack through a lewis acid mechanism.
The crystal structure of the native and peroxide bound form of
VClPO from Curvularia inaequalis have been reported by Messerschmidt,
Prade and Wever (Biol. Chem.,, 1997, 378, 309).
The coordination environment for the active species is based on this
structure and is presented in figure 1. The trigonal bipyramidal vanadium
is coordinated to four non-protein oxygen donors (O2-,
OH-, or OH2) and a histidine. Another nearby histidine
is believed to act as an acid-base catalyst. The crystal structures of two
VBrPOs have also been solved: the dodecameric VBrPO from Corallina
officinalis (Isupov, M.N, et al., J. Mol. Biol., 2000, 299,
1035) and the dimeric enzyme from Ascophyllum nodosum (Weyand et
al., J. Mol. Biol., 1999, 293, 595). While all three
have vastly different gross structures, the active sites are all highly
conserved with the VBrPOs having one more hitidine in the active site than the VClPO.
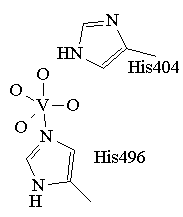
Figure 1. The trigonal bipyrimidal coordination environment
proposed for the active form of VCIPO.

Project Goals
- Use EPR, ESEEM (with the Frasch and LoBrutto groups at Arizona
State), and ENDOR (with the Britt group at UC-Davis) to explore
spectroscopic models for the reduced form of vanadium haloperoxidase
(VHPO) and to acquire a library of data for known compounds so
vanadium(IV) can be more widely used as a spectroscopic probe in other
biological systems.
- Improve on our functional models for VHPO, including
[VO(O2)(Hheida)]- (Scheme 1), which is already the
most efficient model known to date.
- Investigate the amavadin model complex
[V(hida)2]2-, looking for an answer for amavadin's
role in nature.
- Apply the knowledge gained from our research in vanadium chemistry
to broader areas, such as vanadium's insulin mimetic activity.

Current Work
We have recently synthesized and characterized several peroxovanadium
complexes which act as functional models for the vanadium haloperoxidases.
Figure 2 shows the structure of [VO(O2)Hheida]-, one
of these complexes. The distorted pentagonal bipyramidal structure and the
side-on bound peroxo ligand are typical for complexes of this type. In
acetonitrile solution these compounds rapidly and efficiently catalyze the
two-electron oxidation of bromide and iodide upon the addition of acid, as
can be observed either by monitoring the formation of trihalide or by
monitoring the halogenation of organic substrates such as Phenol Red. Upon
the addition of excess hydrogen peroxide in the absence of substrate,
dioxygen is produced via the halide-assisted disproportionation of
hydrogen peroxide.
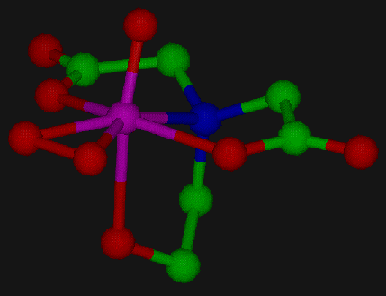
Figure 2. This figure represents the crystallographic structure
of the molecule [VO(O2)Hheida], one of a series of catalytic
models for the vanadium haloperoxidase enzyme.
Kinetic experiments are consistent with a mechanism which is first order
in halide and vanadium complex, and kinetic and mechanistic experiments
reveal that protonation of the complex is essential for the halide
oxidation reaction to occur. Rate constants for halide oxidation and
equilibrium constants for the protonation of the complexes have been
obtained from the kinetic data. A proposed mechanism for the model
compounds studied (and by extension the haloperoxidases) is illustrated in
Scheme 2. This mechanism is consistent with the kinetic and structural
information reported for the enzymes given above, and lends support to the
proposal that an acid/base catalyst is essential to the enzymes'
activity.
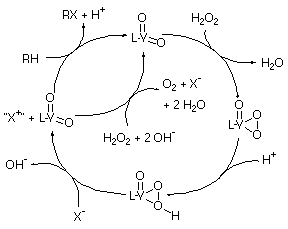
Scheme 2. The catalytic cycle proposed for VHPOs based on our
work.
Current research on these systems focuses on probing the mechanism of
formation of peroxovanadium complexes under these conditions, and on
pinpointing the site of protonation which activates these complexes toward
halide oxidation. We also seek to understand how structural changes in the
complexes affect their reactivity.
On a related note, we have also begun to investigate the structural and
spectroscopic properties of vanadium(IV) complexes with these ligands.
Figure 3 illustrates the crystal structure of one of these complexes. We
believe that a combination of crystallography, UV/visible spectroscopy,
and continuous-wave and pulsed EPR spectroscopies is likely to provide
important insights into the structures of the reduced, inactive forms of
the vanadium-dependent haloperoxidases and other vanadyl-substituted
proteins.
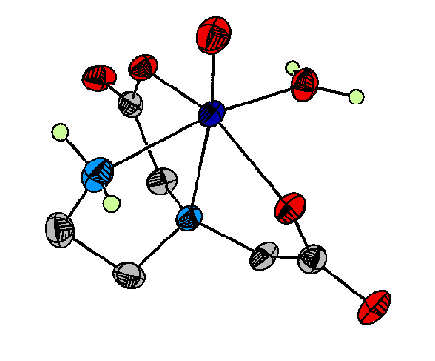
Figure 3. This molecule has been used as a spectroscopic model
for vanadium (IV) binding to proteins such as the chloroplast coupling
factor.
Modern computational methods have made it possible to predict the
configuration and behavior of molecules and complexes enabling us the
better design small molecule models. We are using an active site model
which includes several small molecules which are representative of the
side chains involved in the hydrogen bonding network of the active site.
Upon minimization of the resting structure, we will simulate the
sequential binding of substrates (H2O2, H+,
halide) in various binding modes in order to elucidate their position
and orientation during catalysis and hence which residues are involved
in the reactions.

Additivity Relationship of Vanadyl Complexes in EPR
The additivity relationship for vanadyl complexes is a useful tool
that correlates the hyperfine coupling constant (all) from EPR
spectra to
the types of ligands bound equatorially to vanadium. Unfortunately, use
of this tool has been hampered by ambiguity in the additivity value for
imidazole. To better determine the additivity relationship value for
imidazole, we have synthesized several new vanadyl complexes (such as the
one in Figure 4) with bound imidazole.
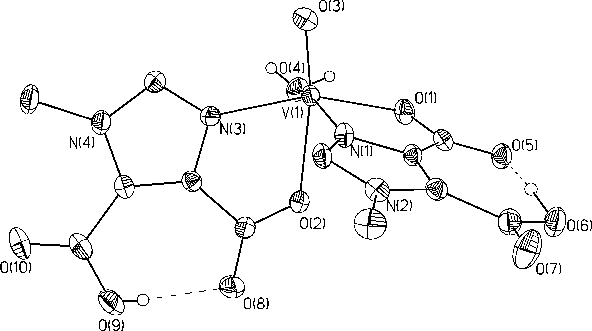
Figure 4. This is one of the molecules which has been used to
determine the additivity relationship which correlates the hyperfine
coupling constant (all) from EPR spectra to
the types of ligands bound equatorially to vanadium.
Together with the four
previously crystallographically characterized vanadyl-imidazole complexes,
additivity values were determined for imidazole. These values fell
predominately into two groups, which correlated to the orientation of the
imidazole ring relative to the vanadyl unit. When the ring makes a small
angle to the vanadyl unit it has a much smaller value (~40 x
10-4 cm-1)
than when it is 90 degrees away (~46 x 10-4 cm-1),
and the data may be fit to a sine curve (See Figure 5). There is also
preliminary evidence that orientation affects the contribution of other
imine-type donors as well.
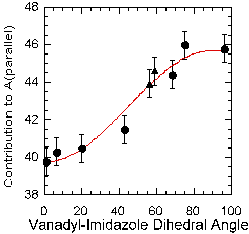
Figure 5. Sine curve fitting of the relationship between the
vanadyl-imidizole dihedral angle and the contribution that imidizole makes
to All

Recent Project Publications (1995-present)
- Dissertations:
- Brent Hamstra, 1997, "Vanadium-Aminocarboxylate
Complexes: Models for the Roles of Vanadium in Biological Systems".
- Thomas S. Smith II, 2001 , "Exploring Reactive and
Spectroscopic
Models of the Vanadium Haloperoxidases"
- Joslyn Yudenfreund Kravitz , 2005 , "Computational Studies
of the Vanadium Dependent Haloperoxidases and Vanadyl-Imidazyol Complexes"
- Undergraduate Thesis:
- Jason Walter Kieltyka, 2000, "Strategies for
Modeling the Vanadium Haloperoxidase".
- Journals, Periodicals, and
Books:
- Colpas, G.C.; Hamstra, B.J.; Kampf, J.W.; Pecoraro, V.L. "Functional
Models for Vanadium Haloperoxidase: Reactivity and Mechanism of Halide
Oxidation" J. Am. Chem. Soc., 1996, 118, 3469.
- Slebodnick, C.; Hamstra, B.J.; Pecoraro, V.L. "Modelling the
Biological Chemistry of Vanadium: Structural and Reactivity Studies
Elucidating Biological Function", Structure and Bonding, P. Sadler,
Ed.,
1997, 89, 57.
- Slebodnick, C.; Hamstra, B.J.; Pecoraro, V.L.; "Modeling the
Biological Chemistry of Vanadium: Structural and Reactivity Studies
Elucidating Biological Function", Metal Sites in Proteins and
Models, 1997, 89, 51-108.
- LoBrutto, R.; Hamstra, B. J.; Colpas, G. J.; Pecoraro, V. L.; Frasch,
W. D.; "ESEEM Spectroscopy Reveals and Distinguishes Equatorial and Axial
Nitrogen Ligands Bound to VO2+"; J. Am. Chem.
Soc., 1998, 120, 4410-4416.
- Hamstra, B. J.; Houseman, A. L. P.; Colpas, G. J.; Kampf, J.W.;
LoBrutto, R.; Frasch, W. D.; Pecoraro, V. L.; "Structural and Solution
Characterization of Mononuclear V(IV) Complexes that Help to Elucidate the
Active
Site Structure of the Reduced Vanadium Haloperoxidases",
Inorg. Chem., 1997, 36, 4866.
- Hamstra, B. J.; Gillis, M.; Colpas, G. J.; Kampf, J. W.; Pecoraro, V.
L.; "An Analysis of the Structural Reorganization Obsereved in V(IV) and
V(V) Aminocarboxylate Complexes: Implications for the Reactivity of V(IV)
and V(V) Complexes with Hydrogen Peroxide", Inorg.
Chem.,
- Hamstra, B. J.; Pecoraro, V. L.; "Reactivity of Dioxovanadium(V)
Complexes with Hydrogen Peroxide: Implications for Vanadium
Haloperoxidases", Inorg. Chem., 1998, 37, 949-955.
- Grant, C.V.; Ball, J.A.; Hamstra, B.J.; Pecoraro, V.L.;
"V51 Studies of Oxovanadium(IV) Complexes: Investigation of
the Nuclear Quadrupole Interaction", Jour. Phys. Chem. B,
1998, 102, 8145-8150.
- Slebodnick, C.; Pecoraro, V.L.; "Solvent Effects on V51
Shifts: Characterization of Vanadate and peroxovanadate Complexes in
Mixed Water/Acetonitrile Solvent", Inorg. Chim. Acta, 1998,
283, 37-43 (Special Issue Devoted to Osamu Yamauchi).
- Thomas S. Smith, II, Charles A. Root, Jeff W. Kampf, Paul
G. Rasmussen, and Vincent L. Pecoraro, "Reevaluation of the Additivity
Relationship for Vanadyl-Imidazole Complexes: Correlation of the EPR
Hyperfine Constant with Ring Orientation", J. Am. Chem. Soc.,
2000, 122, 767-75.
- Smith II, T.S.; LoBrutto, R.; Pecoraro, V.L. "Paramagnetic
Spectroscopy of Vanadyl Complexes: Applications to Biological Systems,"
Coord. Chem. Reviews, 2002, 228, 1-18.
- Smith II, T.S.; Pecoraro, V.L. "Oxidation of Organic Sulfides By Vanadium Haloperoxidase Model Complexes," Inorg. Chem., 2002, 41, 6754-6760.
- Zampella, G; Kravitz, J.Y.; Webster,C. E.;
Fantucci, P.; Hall, M. B.; Carlson, H. A.;
Pecoraro, Vincent L.; Gioia, L. D.; "Quantum Mechanical Models of the
Resting
State of the Vanadium-Dependent Haloperoxidase" Inorg. Chem.,
2004, 43, 4127-4136.
- Zampella, G.; Fantucci, P.; Pecoraro,V.L.;, and Gioia, L. D.;;
"Reactivity of Peroxo Forms of the Vanadium Haloperoxidase
Cofactor. A DFT Investigation"
J. Am. Chem. Soc. 2005, 127, 953-960
- Kravitz, J.Y. and Pecoraro, V. L.; " Synthetic and computational modeling of the vanadium-dependent haloperoxidases", Pure Appl. Chem. 2005 , 77(9), 1595-1605.
- Zampella, G.; Fantucci, P.; Pecoraro,V.L.;, and Gioia, L. D.;; "Insight into the Catalytic Mechanism of Vanadium Haloperoxidases. DFT
Investigation of Vanadium Cofactor Reactivity." Inorg. Chem., 2006, 45(18), 7133-7143.
- Schneider, C. J.; Zampella, G.; Greco, C.; Pecoraro, V. L.; Gioia, L. D.;; "Mechanistic analysis of nucleophilic substrates oxidation
by functional models of vanadium-dependent haloperoxidases: a density functional theory study", Eur. J. Inorg. Chem., 2007, (4), 515-523.
- Schneider, C.J.; Pecoraro, V.L.; "Understanding the Mechanism of Vanadium Dependent Haloperoxidases and Related Biomimetic Catalysis," ACS Symposium Series, 2007, 974,
Chapter 12, 148-162.
- Schneider, C.J.; Penner-Hahn, J.E.; Pecoraro, V.L.; "Elucidating the Protonation Site of Vanadium Peroxide Complexes and the Implications for Biomimetic Catalysis" J. Amer. Chem.
Soc., 2008, 130, 2712-2713.















