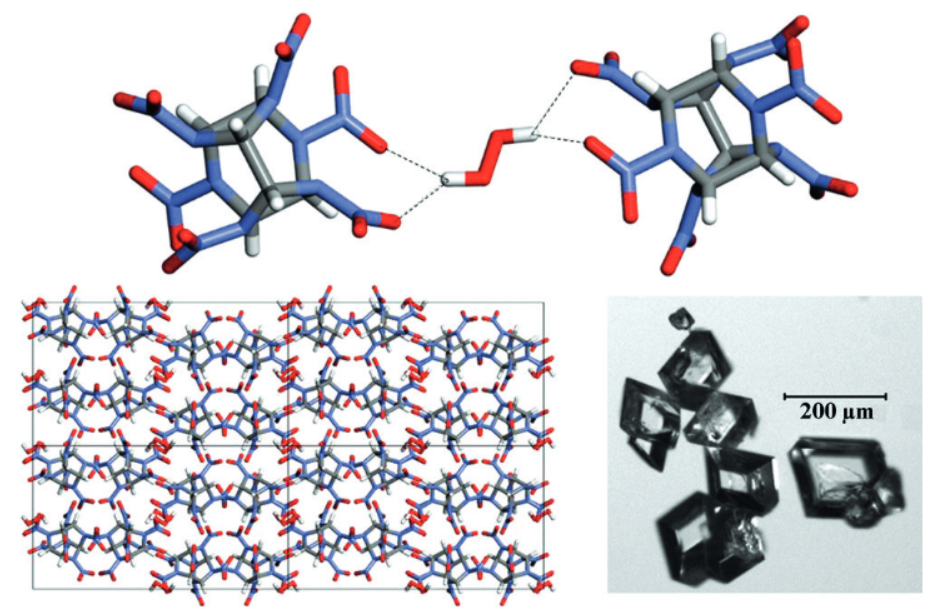
Desirable properties in energetic materials include high density, thermal stability, low hygroscopicity, and low sensitivity; these properties and others are correlated to the crystalline order of the material in the solid state. Cocrystallization, the combination of two or more compounds in the solid state with a defined order and stoichiometry, affords the opportunity to rationally design novel energetics with improved properties and without the challenges and uncertainty of traditional synthesis pathways. Work in this area is fundamental to understanding how interactions in the solid-state manifest in physical properties and will inform the design of myriad future materials ranging from safer air-bag gas generators to more efficient and environmentally benign rocket boosters.
Recent Publications
Bellas, M. K.; Matzger, A. J. "Discovery strategy leads to the first melt-castable cocrystal based on an energetic oxidizing salt." Chem. Sci., 2022 (online)
Bellas, M. K.; Matzger, A. J. "Peroxosolvate discovery method leads to first cocrystal with three energetic components." Chem. Commun., 2022 (online)
Wiscons, R. A.; Nikhar, R.; Szalewicz, K.; Matzger, A. J. "Factors influencing hydrogen peroxide versus water inclusion in molecular crystals." Phys. Chem. Chem. Phys., 2022 (online)
Foroughi, L. M.; Matzger, A. J. "From Hydrate to Peroxosolvate: A Test of Prediction with Cyclic N-Oxides." Cryst. Growth Des., 2021 (online)
Bellas, M. K.; Mackenzie, L. V.; Matzger, A. J. "Lamellar Architecture Affords Salt Cocrystals with Tunable Stoichiometry." Cryst. Growth Des., 2021 (online)
Bennion J. C.; Matzger, A. J. "Development and Evolution of Energetic Cocrystals." Acc. Chem. Res., 2021 (online)
Foroughi, L. M.; Matzger, A. J. "From Hydrate to Peroxosolvate: A Test of Prediction with Cyclic N-Oxides." Cryst. Growth Des., 2021 (online)
|