References
The use of idium metal has recently become very popular in the synthetic reduction of hydroxylamines. The popularity it has achieved has been due mainly to its greater stability in water as apposed to other metals used for similar reductions historically. it's The exact mechanistic pathway by which indium performs this reduction has not yet been mapped out, however, it is commonly believed to act through a single electron transfer mechanism. This paper is cited for its description of the procedure for indium mediated reduction of hydroxylamines.
Reference:
Cicchi, S.; Bonanni, M.; Cardona, F.; Revuelta, J.; Goti, A. Org. Lett. 2003, 5, 1773-1776.
Meaningful papers that also cited the article above:
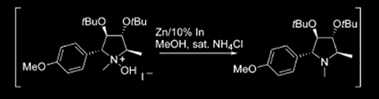
For the following articles, each group of chemists used Indium metal to reduce a hydroxylamine to an amine.
First paper:
Pisani, L.; Superchi, S. Tetrahedron: Asymmetry 2008, 19, 1784–1789.
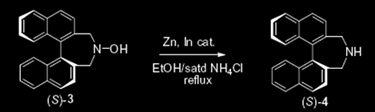
Second Paper:
Moran, J.; Gorelsky, S. I.; Dimitrijevic, E.; Lebrun, M.; Bédard, A.; Séguin, C.; Beauchemin, A. M. J. Am. Chem. Soc. 2008, 130, 17893–17906.
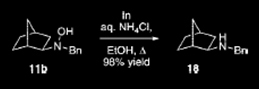
Third Paper:
Goti, A.; Cicchi, S.; Mannucci, V.; Cardona, F.; Guarna, F.; Merino, P.; Tejero, T. Org. Lett. 2003, 5, 4235-4238.