
"HOLLY. Get away from that! We're trying to go undetected here!" Despite Kari's quick admonishment, it was too late. With a thunderous CRASH, Eric and Kari turned to see Holly standing meekly by a broken beaker. "Uhmmm.... it wasn't me?" she suggested timdly. "Hahahah... awwwwwhhh," giggled Eric, amused by Holly's klutziness. "Yeah, it's a long story," explained Kari. "Well, whatever. MUAHAHA. I have you now! There's no hope for escape!" recovered Blofeld, stroking his white Persian cat, Mrs. Whiskers. "Bring them to control room so that they can see my master plan in action!" **Note: This is not meant to suggest that Tony is prone to rambling. We actually think that Tony is an excellent USI and gives very clear and helpful explanations. To any other 215/216 students reading this: Don't tune out Tony. Really. (Also: thanks for agreeing to be our villian, Tony!) Provide a brief explanation for the observed regioselective reduction This reaction uses BF3•Et2O in combination with Et3SiH to reduce one, but not both, of the hydroxyl groups of 55. This regioselection occurs due to the sterics hinderance of one of the groups. The selected OH group is less sterically hindered than the other due to the 3D shape of the seven-membered ring on which it is a substituent. The hydroxyl group that is closest not reduced is sterically hindered by the large size of the nearby bromine atom. This prevents the approach of the bulky boron trifluoride etherate adduct as well as the bulky triethylsilane that donates the hydride after the formation of the double bond. 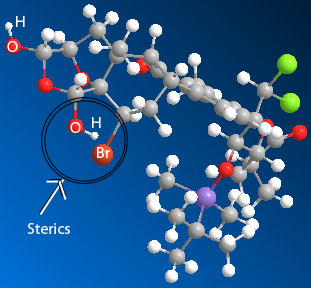 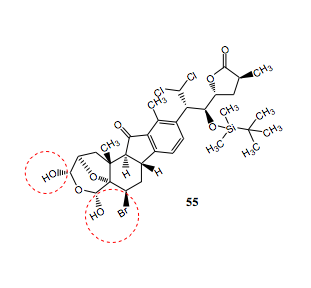
|
Return to Main SSG Page