
 |
 |
 |
 |
 |
 |
 |
Speculate as to what other possibly multistep alternate methods might be used to achieve the same overall transformation from 16 to 17.
An additional method of synthesizing molecule 17 from molecule 16 involves the use of the Wittig reagent and ring closing metathesis. The first step uses the Wittig reagent to remove the double bonded oxygen on molecule 16. The reaction will occur at the bottom of molecule 16 at the C=O because this oxygen has less steric hindrance. This leaves a double bonded carbon and two hydrogens. Next, ring closing metathesis can close the ring, leaving molecule 17. This step requires an M=C catalyst, where M is usually Ru, Mo, W, or Ti. It is key that this M=C pi bond is present under reaction conditions. A commonly used catalyst is the Grubbs catalyst. The reaction will result with a ring closure, leaving molecule 17.
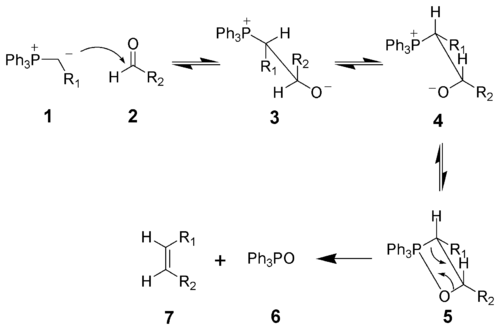
Wittig Reaction
First discovered by Georg Wittig in 1954, the Wittig Reaction is a synthesis method to produce alkenes. This method is normally used to bond aldehydes and ketones to phosphine ylides to form, almost exclusively, the Z alkene product. In the reaction, a carbanion attacks the ketone (or aldehyde) carbon, resulting in (3). Next the phosphorus cation aligns in-plane with the oxygen anion (4), causing the formation of a four-membered ring intermediate (5). A double C=C bond forms (7), leaving Ph3PO (6).
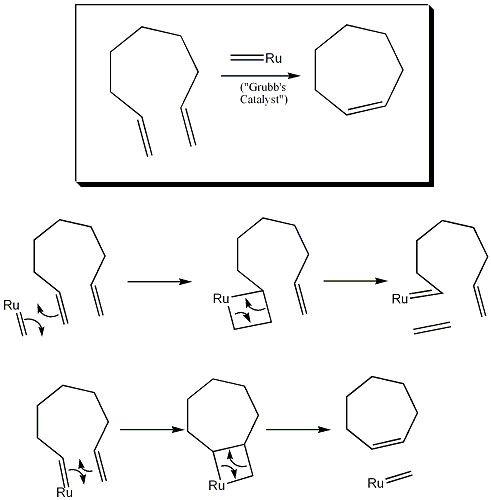
Ring-Closing Metathesis
Ring-Closing Metathesis (RCM) is a process that allows an intramolecular olefin metathesis, which entails closing otherwise difficult-to-make rings. The Grubbs reagent is used. This reagent is a transition metal carbene, typically containing Ru, Mo, W, or Ti. In the reaction, the Grubbs reagent aligns next to a C=C bond on the original molecule (here a nine-membered carbon chain), forming a four-membered ring intermediate. The transition metal (Ru) gets directly bonded to the original molecule. A second four-membered ring intermediate forms, closing the ring by forming a double bond, leaving the transition metal double bonded to an R group.
Grossman, Robert B. The Art of Writing Reasonable Organic Reaction Mechanisms. New York: Springer, 2003.
"Ring-closing Metathesis." Wikipedia, the Free Encyclopedia. Web. 14 Apr. 2011. <http://en.wikipedia.org/wiki/Ring-closing_metathesis>.
"Wittig Reaction." Wikipedia, the Free Encyclopedia. Web. 14 Apr. 2011. <http://en.wikipedia.org/w/index.php?>.