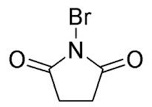
NBS, N-Bromosuccinimide, is a convenient source of cationic bromine due to the electron-withdrawing carbonyl groups. The carbonyl groups on the pentane ring of the molecule make the nitrogen in the N-Br bond partially positive charged through resonance; since nitrogen is more electronegative that bromine, the N-Br bond is polarized so that Br holds much of the positive charge the nitrogen normally would hold. As a result, the bromine is electrophilic and the sulfur in thioglycoside 19 acts as a nucleophile and attacks the Br from NBS. This causes the sulfur of molecule 19 to become positively charged and thus a good leaving group; an SN1 mechanism follows were the brominated sulfur complex leaves due to assistance from the lone electron pairs of the neighboring oxygen. Since molecule 19 then becomes ionic, a H2O molecule attacks the cationic carbon and deprotonation of one of the H’s of H2O yields an alcohol intermediate.
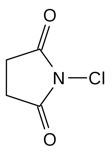
Other reagents could be used for this same transformation; four notable reagents include Br2, Cl2, NCl (N-Chlorosuccinimide), and NIS (N-Iodosuccinimide). All four reagents have an electrophilic halogen that the nucleophilic sulfur could attack. Br2 and Cl2 are particularly reactive because their molecules are highly polarizable, so when they approach an electron source, one end becomes negatively charged while the other becomes positively charged. Br2 is somewhat even more reactive than Cl2 because it is larger and thus more polarizable, so if used in this transformation, care must be taken that nucleophilic attack on bromine. Although NIS is used as a source of electrophilic iodine in many reactions, iodine is not the best choice for adding onto sulfur in molecule 19; out of the four reagents, NIS is least effective. This efficiency is due to the p-orbital sizes of iodine and sulfur. P orbitals of similar sizes interact most efficiently and are able to donate electrons to one-another most effectively. Iodine, which has two additional electron shells compared to sulfur, does not accept sulfur’s electrons as quickly as bromine or chlorine. The atomic orbitals of chlorine interact easily with those of sulfur’s since both of their orbitals are similar in size. As a result, chlorinating the sulfur is also an effective way of making sulfur into a good leaving group that will leave via lone electron pair assisted ionization.

References:
Ghosh, S.; Misra, A. K. Tetrahedron: Asymm. 2010, 21, 2755-2761.
Yilmaz, S. S.; Abbasoglu, R. J. of Molec. Modeling 2006, 12, 290-296