Completo, G. C.; Lowary, T. L. J. Org. Chem. 2008, 73, 4513-4525.
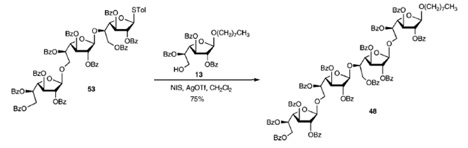
Throughout this paper, multiple glycosylation products were produced through the use of N-Iodosuccinimide (NIS) and silver triflate (AgOTf) activation. For example, in the following reaction of 53 to 28, the thiogycoside 53 is activated using NIS–AgOTf in the presence of octyl glycoside acceptor 13, giving the desired tetrasaccharide 48 in 75% yield.
Glycosylation is a process that attaches a glycan (such as a monosaccharide or oligosaccharide) to an organic molecule. The authors states that molecule 13 is the glycosyl acceptor. By convention, the term “glycosyl acceptor” means that molecule 13 is the nucleophile in the reaction, with oxygen typically as the nucleophilic atom. In contrast, a “gycosyl donor” typically contains the carbon that becomes anomeric after the transformation.
In the transformation from 53 to 48, molecule 13 is able to attach to molecule 53 because of the used of NIS. NIS is an analog of the NBS used in our reaction scheme converting 19 to 20. In Completo’s and Lowary’s paper, NIS is used to iodinate the sulfur in molecule 53, in order to create a positive charge on sulfur. This converts the STol group into a good leaving group. The sulfur-carbon single bond breaks because of one-pair assisted ionization from the oxygen that is two bond neighbor to sulfur. Glycosylation is often highly regio- and steroselective so that one product is formed rather than an anomeric mixture.