Task 2

Task 2

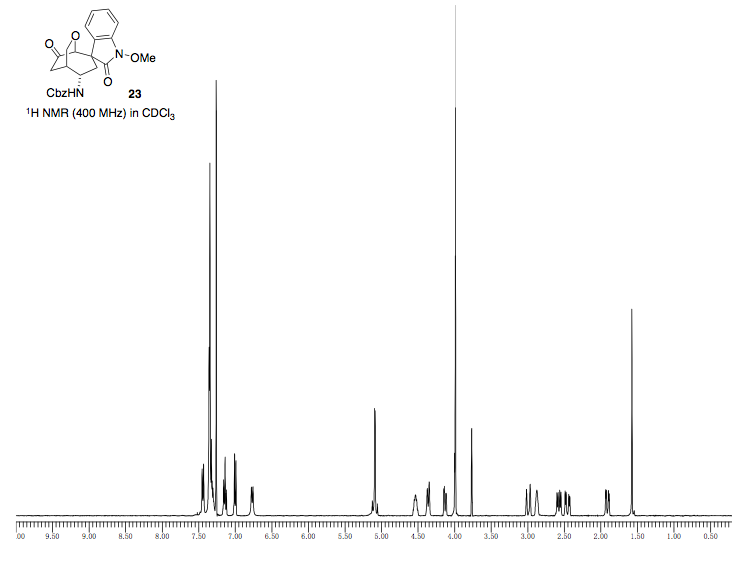
MOLECULE 23
| Label | Chemical Shift | Integration | J (Hz) | Multiplicity | Explanation |
| a | 7.00 | 1H | 7.8 | d | This H is attached to an aromatic ring and also experiences coupling with H(b) to give a doublet. |
| b | 7.14 | 1H | 7.6, 7.6 | dd | This H is attached to an aromatic ring and also experiences coupling with H(a) and H(c) to give a doublet of doublets. |
| c | 7.40-7.27 | 1H | - | m | This H is attached to an aromatic ring and also experiences coupling with H(b) and H(d) to give a multiplet. |
| d | 7.44 | 1H | 7.3 | d | This H is attached to an aromatic ring and also experiences coupling with H(c) to give a doublet. |
| e | 3.77 | 3H | - | s | This is a methoxy group methyl that has 3 equivalent H's and experiences no coupling. |
| f | 2.57 | 1H | 15.6, 5.5 | dd | This H is attached to a carbon and experiences strong coupling with H(g) and weaker coupling with H(m) resulting in a doublet of doublets. |
| h | 2.88 | 1H | - | m | This H has many couplings, a multiplet. It is attached to a carbon which is attached to a ketone. It couples with H(i), H(k), and "W" coupling with H(n). |
| i | 2.99 | 1H | 19.7 | dd | This H is near a ketone which de-shields the H slightly, and has strong diastereotopic coupling with H(h). |
| j | 4.36 | 1H | 10.5 | d | This H has coupling interactions with H(n) and is attached to a C-O carbon. |
| k | 2.88 | 1H | - | m | This H has many couplings, resutling in a multiplet. It is attached to a carbon which is attached to all carbons as well, so it has relatively lower chemical shift. |
| l | 3.99 | 1H | - | s |
This H experiences no coupling and is attached to a C-O carbon, making it shift farther downfield. |
| m | 4.54 | 1H | - | m | This H is attached to a C-N carbon, making it shift relatively downfield. This H couples with H(k), H(g), and H(f). |
| p | 6.77 | 1H | - | s | This H is very downfield, and it is a bit broader at the top, indicating that it could be bound to the N. |
| n | 4.13 | 1H | 10.5, 3.2 | dd | This H has coupling interactions with H(j) and experiences "W" coupling with H(h). It is also attached to a C-O carbon. |
| g | 2.46 | 1H | 15.6, 6.4 | dd | This H is attached to a carbon and experiences strong coupling with H(f) and weaker coupling with H(m) resulting in a doublet of doublets. |
| r | 7.40-7.27 | 2H | - | m | This H is attached to an aromatic ring and also experiences coupling with other aromatic H’s to give a multiplet. |
| s | 7.40-7.27 | 2H | - | m | This H is attached to an aromatic ring and also experiences coupling with other aromatic H’s to give a multiplet. |
| t | 7.40-7.27 | 1H | - | m | This H is attached to an aromatic ring and also experiences coupling with other aromatic H’s to give a multiplet. |
| u | 5.07 | 1H | 12.4 | d | This H is attached to a carbon which is also attached to a benzene ring and an oxygen. It also shows a strong coupling interaction with H(v). |
| v | 5.11 | 1H | 12.4 | d | This H is attached to a carbon which is also attached to a benzene ring and an oxygen. It also shows a strong coupling interaction with H(u). |