Glossary:
Flash Chromatography
Flash chromatography is a purification method used to separate mixtures.
1. In the bottom of the burrett, add 1cm steel wool and 1cm sand on top of the steel wool
2. Add silica gel (silica with a little ethyl acetate) to fill half of the column
3. Pour 1 cm more sand on top silica
4. Load compound dissolved in as little organic solvent as possible
5. Begin pouring mixture of ethyl acetate/hexane or other solvent system, ratio
determined by TLC previous to column with goal of .3 Rf
6. Collect vials of product and run TLC of vials to determine if a product is present
7. After both products have stopped showing up on TLC plates, flush out silica and keep
vials separated, run NMR or IR for classification of the pure products

Gravity Filtration (Filtration)
Gravity filtration is used to filter a solid from a solution where the goal is to keep the solution.
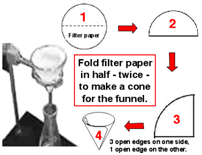
1. Fold filter paper to fit in a funnel
2. Insert funnel with filter into the top of an erlenmeyer flask
3. Pour solution with solid into the filter and let the solution filter through, keeping the
solid trapped in the filter
Rotary Evaporator (Rotavap)
A rotovap is device used in chemical laboratories to remove solvents from samples by evaporation.

1. A motor unit that rotates the evaporation flask or vial containing the user's sample.
2. A vapor duct that is the axis for sample rotation, and is a vacuum-tight conduit for the
vapor being drawn off of the sample.
3. A vacuum system, to substantially reduce the pressure within the evaporator system.
4. A heated fluid bath (generally water) to heat the sample.
5. A condenser with either a coil passing coolant, or a "cold finger" into which coolant
mixtures such as dry ice and acetone are placed.
6. A condensate-collecting flask at the bottom of the condenser, to catch the distilling
solvent after it re-condenses.
7. A mechanical or motorized mechanism to quickly lift the evaporation flask from the
heating bath.
Magnesium Sulfate (MgSO4)
Water is extremely difficult to remove from chemical compounds because solvents usually hold onto it well. In general, the more polar a solvent is, the more water it will hold. Drying agents like magnesium sulfate (MgSO4) work by complexing with H2O in the solvent and forming a hydrated MgSO4 precipitate. This precipitate can then be gravity filtered out, yielding an anhydrous product. Compared to other common drying agents, MgSO4 is slightly acidic, works fairly quickly, and works better in diethyl ether compared to ethyl acetate.