Mulan's fellow soldiers have been imagining their dream girls, but Mulan just told them that what their future wives are really looking for--a man who can answer all types of questions. Ling, Chien-Po, and Yao were doing well until they reached the question below. Look at their answer! (Remember they're desperate to get their dream girls, so they put in a lot of effort to answer this question)
Question: How is scandium tris (triflurormethanesulfonate) used in organic synthesis?
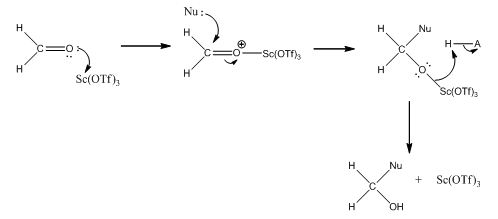
- Aldehydes are commonly involved in nucleophilic addition reactions with allylsilanes. However, allylsilanes have an inherently low nucleophilicity, making these reactions difficult to proceed in normal conditions. In this case, a Lewis acid catalyst is required to activate the aldehyde in order for the allylsilane to be able to undergo a nucleophilic attack. Metal based Lewis acids such as scandium trifluoromethanesulfonate are highly oxophilic, and form a strong but easily altered bond with oxygen atoms. Moreover, they allow the reaction to be carried out in mild conditions with high yields of the final product. When scandium trifluoromethanesulfonate is used in nucleophilic addition reactions, it bonds with the oxygen atom in aldehydes, activating the molecule by making the central carbon more electrophilic. This allows reactants with low nucleophilicities to attack and create a new carbon-carbon bond. The bond between the oxygen scandium trifluoromethanesulfonate eventually breaks, and the Lewis acid leaves, reforming the catalyst for subsequent reactions.