H-NMR Correlation
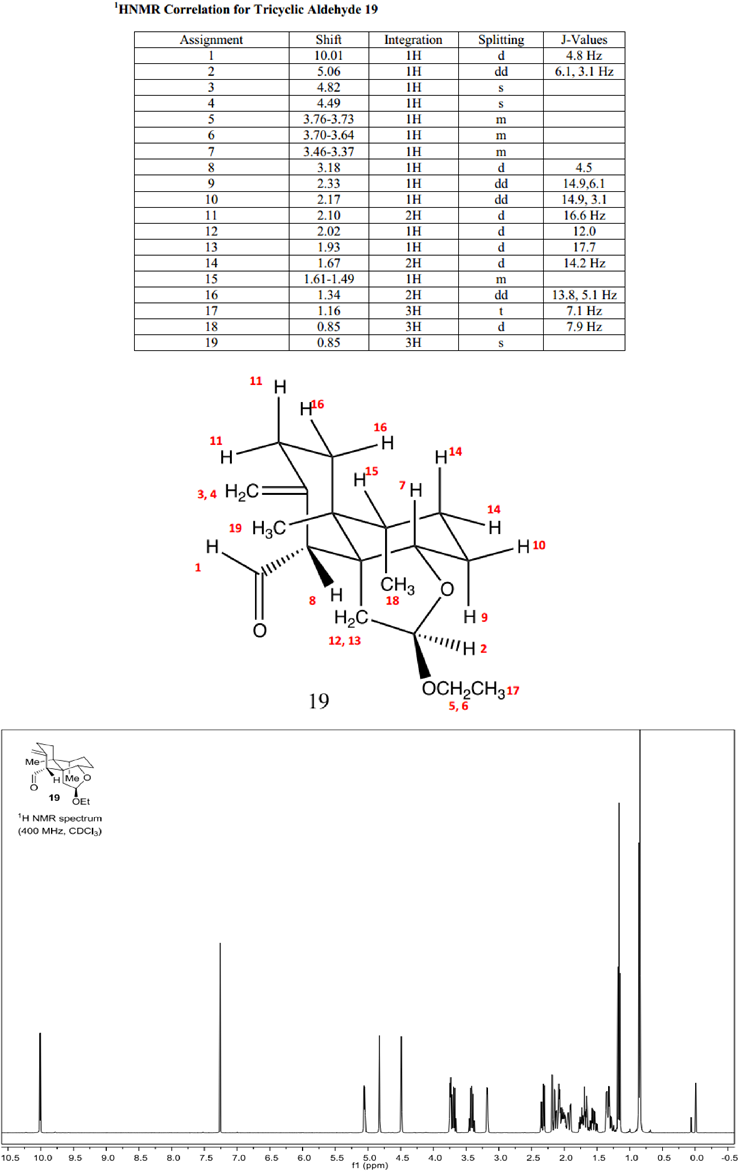
1: The peak at 10.01 ppm corresponds to a hydrogen atom bonded directly to a carbonyl carbon, so it is shifted very far upfield.
2: The peak at 5.06 ppm corresponds to a hydrogen atom bonded to a carbon bonded to two oxygen atoms, so it is shifted moderately upfield.
3, 4: The peaks at 4.82 and 4.49 ppm correspond to two hydrogen atoms bonded to an alkenic carbon resulting in them being shifted moderately upfield.
5, 6: The peaks at 3.76-3.73 and 3.70-3.64 ppm both correspond to hydrogen atoms that are bonded to a carbon bonded to a single oxygen atom. They show up as separate peaks because they are diastereotopic to each other.
7: The peak is at 3.46-3.37 ppm corresponds to a hydrogen atom that is bonded to a carbon bonded to a single oxygen, so it is shifted slightly upfield.
8: The peak at 3.18 ppm corresponds to a hydrogen atom that has only one neighbor, so it shows up as a doublet. The carbon it is bonded to is bonded to two sp2 carbons, so the hydrogen is shifted slightly upfield.
9, 10: The peaks at 2.33 and 2.17 ppm correspond to hydrogen atoms are both neighbors to hydrogen 7 as indicated by coupling values. They have a 14.9 Hz coupling between themselves, and hydrogen 9 has a larger second coupling value because it is axial to hydrogen 7.
11, 14, and 16: The peaks at 2.10, 1.67, and 1.34 ppm each correspond to pairs of hydrogen atoms that show up together. Their distance from various electronegative groups (oxygen and sp2 carbon) were used determined their shift.
12 and 13: The peaks at 2.02 and 1.93 ppm correspond to hydrogen atoms on the same carbon. These hydrogen atoms show up at separate ppm values because of their distance from oxygen differs.
15: The peak at 1.61-1.49 ppm corresponds to a hydrogen atom that is not near any electronegative groups, so it is fairly downfield. It appears as a multiplet because it has two 3-bond coupling neighbors as well as a W-coupling neighbor.
17: The peak at 1.16 ppm has an integration value of 3, and is a triplet indicating it has two neighbors. It is further upfield than other methyl groups because of its proximity to an oxygen atom.
18: The doublet peak at 0.85 ppm has an integration value of three. It corresponds to a methyl group whose hydrogen atoms have only one 3-bond neighbor.
19: This singlet peak at 0.85 ppm has an integration value of three. It corresponds to a methyl group bonded to a carbon atom with no hydrogen atoms.






