Home || Mechanism || HNMR|| Experimental || References ||Leading Questions || About Us || SSG Home
Leading Question:
The Barton-McCombie Deoxygenation

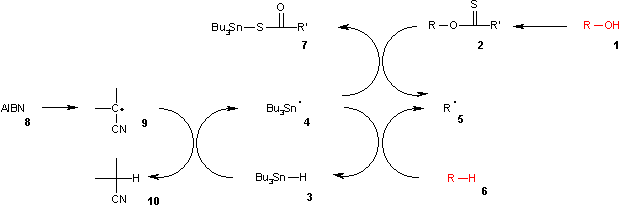
The Barton–McCombie deoxygenation is an organic reaction in which an hydroxy functional group in an organic compound is replaced by a hydrogen to give an alkyl group. The alcohol (1) is first converted into a xanthate (2), which is a salt formula made of ROCS2-M+ (R = alkyl; M+ = Na+, K+). Then, tributylstannane (3), an organotin compound based on tin with hydrocarbon substituents, is decomposed by AIBN (8), which eliminates a molecule of nitrogen gas to form two 2-cyanoprop-2-yl radicals into a tributylstannyl radical (4). The tributyltin radical abstracts the xanthate group from leaving an alkyl radical and tributyltin xanthate (7). The sulfur tin bond in this compound is very stable and provides the driving force for this reaction. The alkyl radical in turn abstracts a hydrogen atom from a new molecule of tributylstannane generating the desired deoxygenated product (6) and a new radical species ready for propagation.