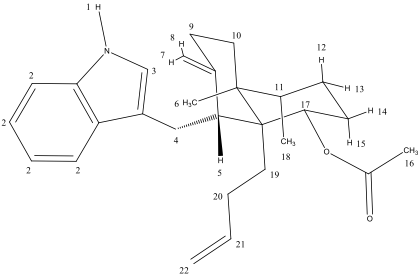
Acetate 24 H-NMR
|
Assignment |
Shift (ppm) |
Integration (H) |
Splitting Pattern |
J-Values (Hz) |
| 1 | 7.93 | 1 | Broad Singlet | N/A |
| 7 | 7.64 | 1 | Doublet | 7.7 |
| 8 | 7.33 | 1 | Doublet | 7.9 |
| 15 | 7.21-7.15 | 1 | Multiplet | N/A |
| 12 | 7.15-7.10 | 1 | Multiplet | N/A |
| 3 | 6.95 | 1 | Singlet | N/A |
| 21 | 5.84-5.70 | 1 | Multiplet | N/A |
| 5 | 5.65 | 1 | Singlet | N/A |
| 14 | 4.98 | 1 | Singlet | N/A |
| 22 | 4.97 | 2 | Doublet of Doublets | 29.2, 13.9 |
| 13 | 4.86 | 1 | Singlet | N/A |
| 17 | 3.31-3.20 | 1 | Multiplet | N/A |
| 4 | 3.13-3.06 | 2 | Multiplet | N/A |
| 11 | 2.50-2.40 | 1 | Multiplet | N/A |
| 9 | 2.33-2.18 | 2 | Multiplet | N/A |
| 16 | 2.09 | 3 | Singlet | N/A |
| 10 | 1.83-1.70 | 2 | Multiplet | N/A |
| 2 | 1.67-1.49 | 4 | Multiplet | N/A |
| 20 | 1.40-1.30 | 2 | Multiplet | N/A |
| 19 | 1.30-1.25 | 2 | Multiplet | N/A |
| 6 | 1.00 | 3 | Singlet | N/A |
| 18 | 0.85 | 3 | Doublet | 6.5 |
1: The peak at 7.93 ppm corresponds to the hydrogen directly attached to nitrogen. It is the most deshielded because of the inductive effect from the nitrogen.
2: The peak at 1.67 is a series of multiplets corresponding to aromatic hydrogens. The 2-bond and 3-bond coupling it has with its neighbors contribute to its appearance as a multiplet.
3: The peak at 6.95 ppm corresponds to the hydrogen adjacent to nitrogen. It shows up as a singlet because it has no vicinal neighbors. This is the most deshielded singlet because of the inductive effect from nitrogen and bonding with the sp2 carbon.
4: The hydrogens in the methylene group between both sets of rings correspond to the peaks from 3.06-3.13. They show up as multiplets because they each have one three-bond neighbor and one two-bond neightbor.
5: At 5.65 ppm, the peak correlates to a single hydrogen. This hydrogen is at a chiral center where there are no other hydrogens within three bonds of it.
6: The peak at 1.00 ppm correlates to a methyl group.
7,8: The peaks at 7.33 and 7.64 ppm correspond to the hydrogens attached to the alkene. They show up as a doublets because they each have one two-bond neighbor with coupling values of 7.7 and 7.9 Hz.
9: The peaks at 2.33-2.18 correlate to two hydrogens. They show up as a multiplet because they are neighbors to an alkene and two other hydrogens attached to a carbon.
10: Similar to the peak labelled 9, these two hydrogens have many neighbors interacting as 2-bond and 3-bond neighbors.
11: The peak at 2.50 is correlated to a methyl group labelled as a multiplet because of its position in the ring it interacts with many other hydrogens.
12: This is a single hydrogen peak at around 7.15. Since it is positioned on the ring in an axial position it has many registering interactions with other hydrogens.
13: This is the single hydrogen peak correlated to 4.86 ppm. It is a singlet because of its axial position.
16: The peak at 2.09 ppm corresponds to the methyl hydrogen atoms of the ester. This methyl singlet is more deshielded than the other methyl singlet because of the inductive effect from the oxygen atoms.
18: The peak at 0.85 ppm corresponds to the methyl hydrogen atoms with one vicinal neighbor. They show up as a doublet because they have one 3 bond hydrogen neighbor.
21: The peak at 5.84 corresponds to one hydrogen attached to an alkene. We see this show up as a multiplet because of the 2-bond and 3-bond coupling it has with its neighbors.
22: The peak at 4.97 ppm corresponds to the germinal hydrogen atoms of the alkene. They show up as a doublet of doublets because their spins couple with the vicinal hydrogen neighbor and each other.