








Synthesis of 12’: N-Bromosuccinimide (NBS) (58 mg, 0.32 mmol) and benzoyl peroxide (5 mg, 0.32 mmol) were added to a solution of compound 2c (80 mg, 0.21 mmol) in 10 mL of CCl4 at room temperature. The mixture was refluxed
Reflux is an experimental method that involves heating a reaction mixture until it reaches the boiling point of the reaction solvent, and the reflux condenser causes the liquid to condense and return to the reaction mixture. This allows reactions to run for long periods of time without the addition of more solvent. The setup is a round-bottomed flask attached to a reflux condenser that is connected to a water line. As the vapor rises up through the condenser, the cold water running through it will cause it to condense and drip back into the reaction mixture.
for 2 hours and then cooled to room temperature. The solvent was removed under vacuum, and the residue was purified using flash chromatographyFlash column chromatography uses compressed air to push the solvent through the column, resulting in better separation in a shorter period of time. A column with glass wool at the bottom is packed with a silica slurry. A thin layer of sand is added, and the sample is loaded on top of the packed column. The column is filled to the top with solvent, and pressure is applied to the column to push the solvent through the column to collect the fractions. The solvent should always be above the top of the silica. (1:15 EtOAc:hexanes) to give compound 12’ (88 mg, 90%) as a white solid. 1H NMR (500 MHz, CDCl3): δ 6.66 (t, J = 8.0 Hz, 1H), 6.50 (s, 1H), 6.48 (d, J = 8.1 Hz, 1H), 5.75 (t, J = 8.7 Hz, 1H), 5.53 (dd, J = 10.1 Hz, J = 2.2 Hz, 1H), 4.85 (d, J = 12.6 Hz, 1H), 4.70 (d, J = 12.6 Hz, 1H), 4.44 (s, 1H), 4.02 (s, 1H), 2.95 (dd, J = 14.5 Hz, J = 3.9 Hz, 1H ), 2.13 (s, 3H), 2.06 (d, J = 14.0 Hz, 1H), 1.65 (s, 3H), 1.16 (s, 3H), 1.15 (s, 3H).
Synthesis of 12: TEMPO (304 mg, 1.95 mmol) and tri-n-butyltin hydride (434 μL, 1.56 mmol) in benzene (10 mL) were added to a solution of compound 12’ (88 mg, 0.19 mmol) in 10 mL of benzene over 30 minutes at 80 ℃. The mixture was refluxed for 2 hours, cooled to room temperature and filtered through a plug of silica gel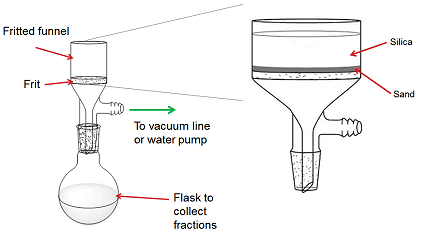
As an alternative to a full column, purification with a silica plug is usually used for samples with high Rf values and few impurities that have very low Rf values. The setup involves placing silica on top of a layer of sand in a fritted funnel connected to a vacuum line. Fractions collected using this method are much larger than those of a column, so a round-bottomed flask is attached to the funnel to collect these fractions. The flask should at most be half-filled; otherwise there is a risk of losing some of the fraction through the vacuum line.. The solvent was removed under vacuum, and the residue was purified by flash chromatography (1:20 EtOAc:hexanes) to give compound 12 (77 mg, 75%) as a white solid. 1H NMR (500 MHz, CDCl3): δ 6.67 (t, J = 8.0 Hz, 1H), 6.49 (d, J = 7.7 Hz, 1H), 6.23 (dd, J = 10.5 Hz, J = 1.2 Hz, 1H), 6.05 (s, 1H), 5.47 (dd, J = 10.5 Hz, J = 1.9 Hz, 1H), 4.78 (d, J = 12.5 Hz, 1H), 4.74 (d, J = 12.8 Hz, 1H), 4.32 (s, 1H), 4.03 (t, J = 6.0 Hz, 1H), 2.97 (ddˈJ = 14.5 Hz, J = 4.7 Hz, 1H ), 2.13 (s, 3H), 2.07 (d, J = 14.5 Hz, 1H), 1.64 (s, 3H), 1.56 (s, 3H), 1.51-1.26 (m, 8H), 1.17 (s, 3H), 1.13 (s, 6H), 1.06 (s, 6H), 1.04 (s, 3H).



