Asymmetric Total Synthesis of Batzelladine D: Molecule 18 to Molecule 23
Cohen, F.; Overman, L. E.; Ly Sakata, S. K. Org. Lett. 1999, 1, 2169-2172.
Cohen, F.; Overman, L. E.; Ly Sakata, S. K. Org. Lett. 1999, 1, 2169-2172.
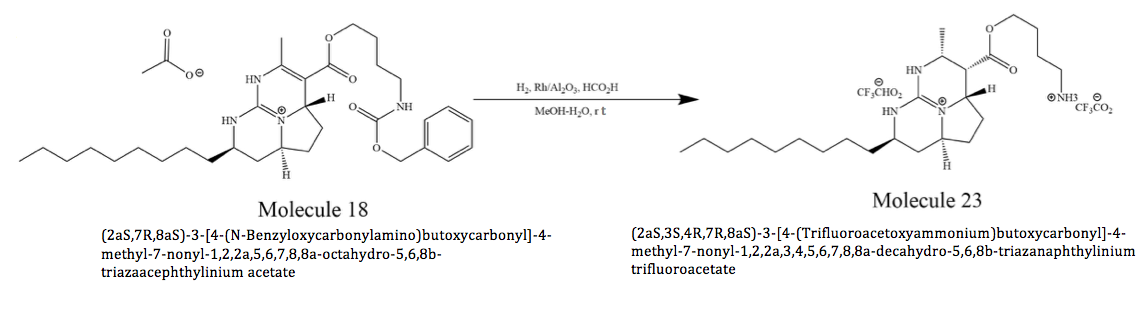


 protein association. In addition, batzelladines have the potential as an anti HIV compound, making them very important to society. Particularly, the researchers modified a tethered Biginelli strategy to prepare (–)-batzelladine D from the same
protein association. In addition, batzelladines have the potential as an anti HIV compound, making them very important to society. Particularly, the researchers modified a tethered Biginelli strategy to prepare (–)-batzelladine D from the same