Of course, you correctly chose "Branched N-1 substituted thymine derivative" Bob! Bob is an important precursor of biological nucleosides, while Stefan can't even wear a shirt.
In the real world, however, regioselective amination to the branched nucleoside is more complex than choosing between Bob and Stefan. Here we will discuss diastereoselectivity control during this amination reaction.
"Branched N-1 Substituted Thymine Derivative" Bob
Leading Question:
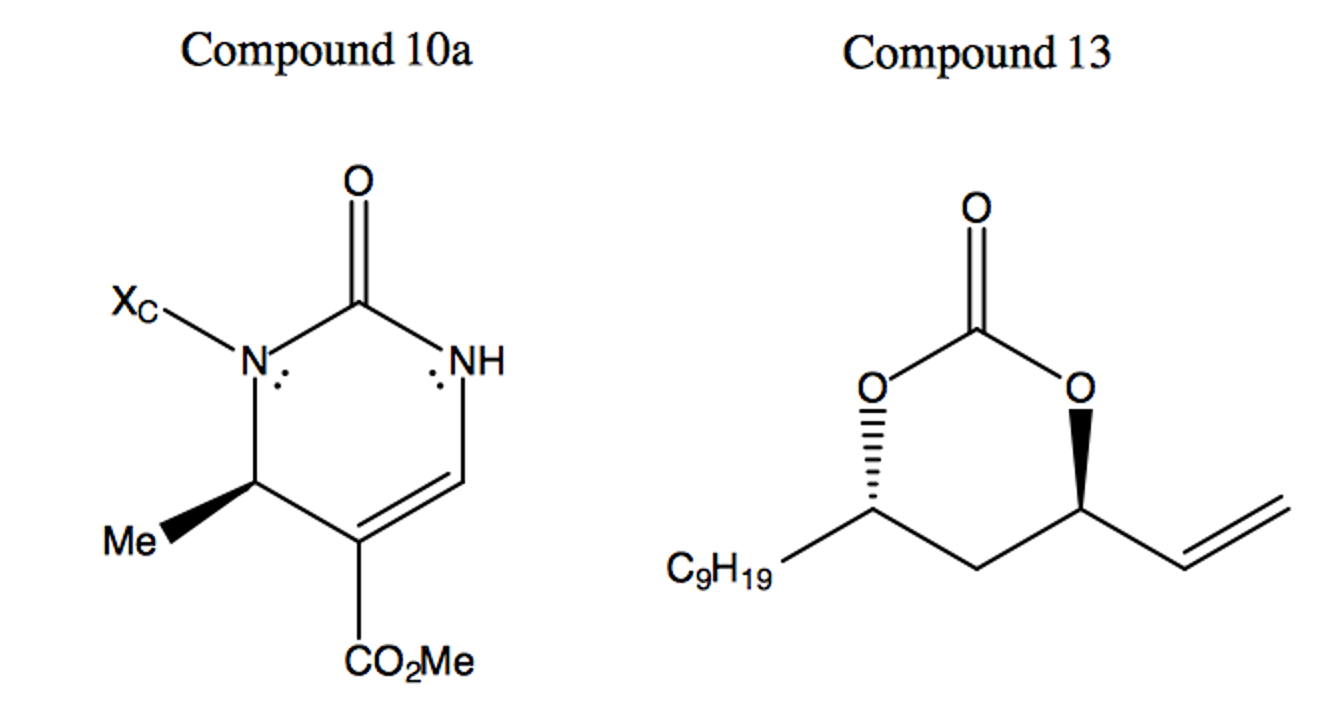
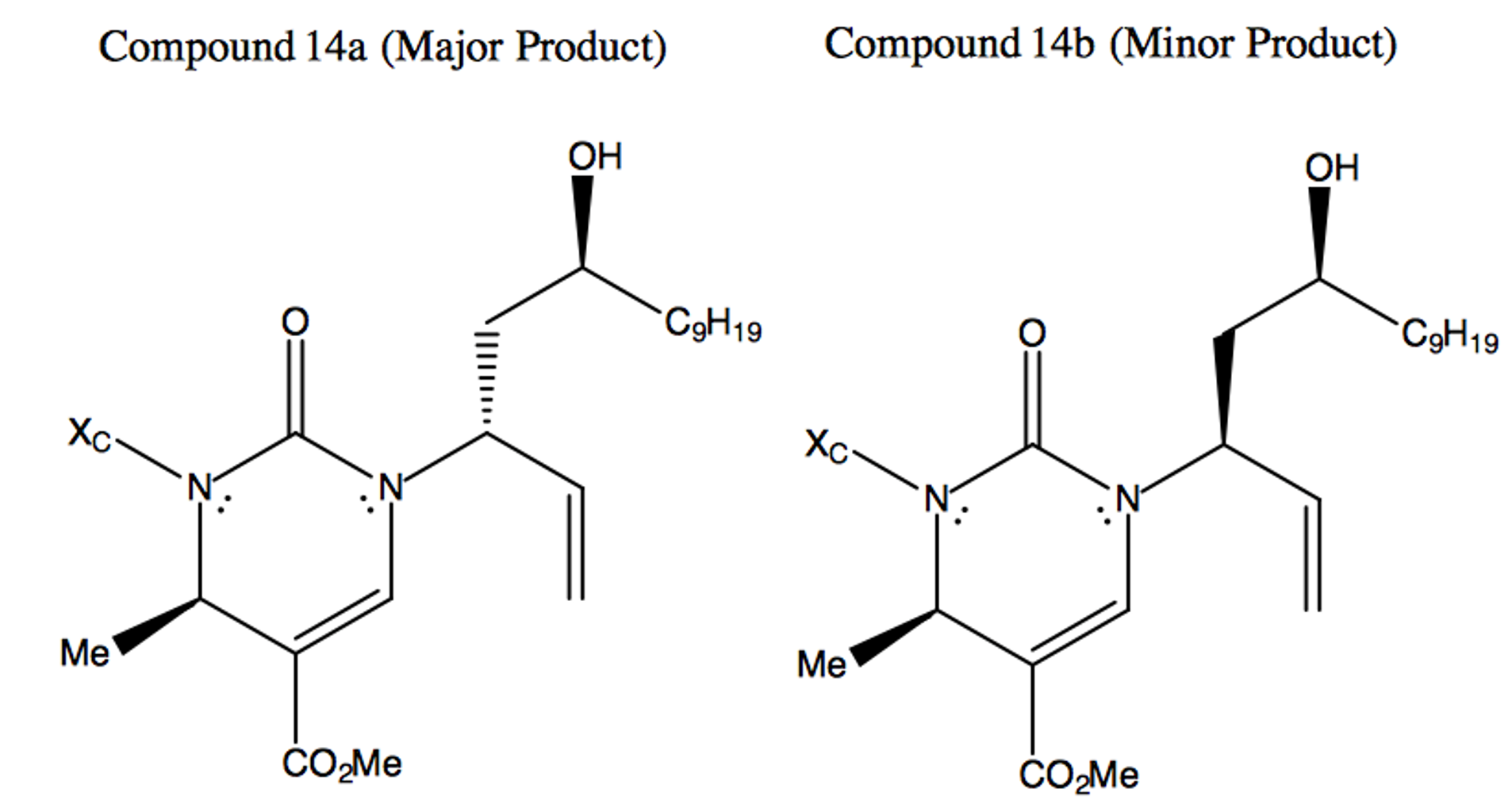
The Rhodium-catalyzed reaction of compound 10a to compound 14 results in a mixture of diastereomers. This combination of isomers favors the R conformation around the original electrophilic reaction site of compound 13 in a ratio of 30:1 over the S conformation. Why is this diastereoselectivity observed? Explain how the reaction mechanism of the Rhodium-catalyzed materials results in this 30:1 mixture of diastereomers.
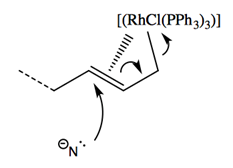
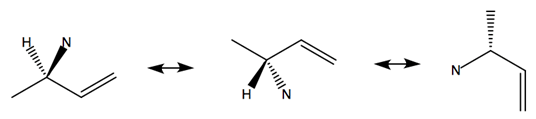
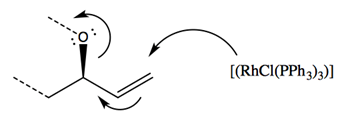
In this reaction, a modified version of Wilkinson’s Catalyst ([RhCl(PPh3)3]) acts on the double bonded carbon branch on compound 13, moving the double bond to the target carbon site and initiating an electron redistribution throughout the molecule. This initial catalyst attack acts in a very quick SN1 reaction, inverting the stereochemistry of the target site. The now-carbon-deficient Rhodium partially shares the adjacent double bond orbitals, creating a partial positive charge on the target carbon site and allowing the nucleophilic Nitrogen on compound 10a attack the electrophilic carbon site in a second SN2 reaction.
The conformation of the electrophilic carbon before catalyst attack is such that the Oxygen (in the pictured frame of reference) comes out of the plane in an R configuration. As the catalyst attacks from the opposite side of the target site relative to the Oxygen, the C-O bond breaks and the stereochemistry of the site is inverted. The new site is promptly attacked by the nucleophilic Nitrogen, reverting the stereochemistry of the target site back to the original R configuration. The minor product is formed from the rare reaction in which the nucleophilic Nitrogen attacks before the Rhodium catalyst shares the double bond’s electrons. In such situations, an SN1 reaction is carried out, with half of the produced molecules exhibiting the form of the minor product.