For more dank supporting articles...

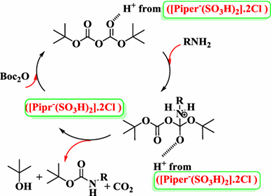
In this paper, researchers attempt to reproduce the results an earlier paper reported about a procedure for chemoselective mono-N-Boc protection of amino acids, amines, and peptides with a di-tert-butyl decarbonate. The reaction was catalyzed with Amberlyst-5 catalyst in ethanol, which can be used for both N-boc protection and deprotection. This experiment involved reacting aniline with 1.1 equiv (Boc)2O with the Amberlyst-15 catalyst present at room temperature. Protic solvents were found to be most effective, particularly ethanol, so it was used as the reaction solvent and generated a respectable yield. A solvent-free reaction was also done to test an earlier report that said this reaction would finish within 1-12 minutes, but results were not consistent with it because the time and experimental yield did not match it. In the reaction with ethanol solvent, no impurities, such as urea or N,N-di-Boc derivatives, were detected from TLC and H1 NMR analyses—a good result. Amines reacted well to give outstanding yields of N-boc products. The researchers concluded that this procedure, taking place at room temperature in ethanol solvent with easily recoverable Amberlyst-5 catalyst, is an efficient procedure for N-tert-butoxycarbonylation of various types of amines.