Experimental

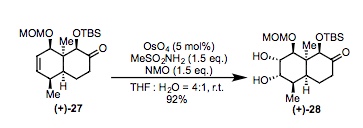
Synthesis of Compound 28 from Compound 27: 1.05 g of compound (+)- 27 (2.85 mmol) was dissolved in 36 mL of THF. Then, N-methylmorpholine-N-oxide (500 mg, 4.227 mmol)and MeSO2NH2 (388 mg, 4.27 mmol) were added. Following this, OsO4 was added (67 mL, 0.14 mmol, 0.5% dilution in water) via a syringe followed by 9 mL of H2O. The reaction mxture was stirred at room temperature overnight before quenching with a saturated solution of Na2S2O3. The reaction mixture was then extracted with Ethyl acetate three times and the combined organic layers were dried over anydrous Na2SO4, filtered and concentrated. Flash chromotography (3:1 hexanes/Ethyl Acetate) resulted in compound(+)-28 (1.06 g, 2.63 mmol, 92% yield) as a white foam.
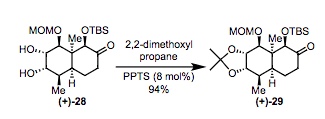
Synthesis of Compound 29 from Compound 28: A solution of 28 (1.02 g, 2.54 mmol) in 2,2-dimethoxypropane (10mL) and dichloromethane (10 mL) and p-toluenesulfonate (PPTS, 50 mg, 0.20 mmol) was added to a reaction flask. The reaction mixture was stirred at room temperature and monitored via TLC until the substrate 28 was fully converted. Then NaHCO3 (180 mg) was added followed by 30 mL hexanes. The resulting suspension was filtered through Celite and then concentrated. Flash chromatography (10:1 hexanes/ Ethyl Acetate) resulting in (+)-29 (1.05 g, 2.37 mmol, 94%) as a white foam.