Mechanism

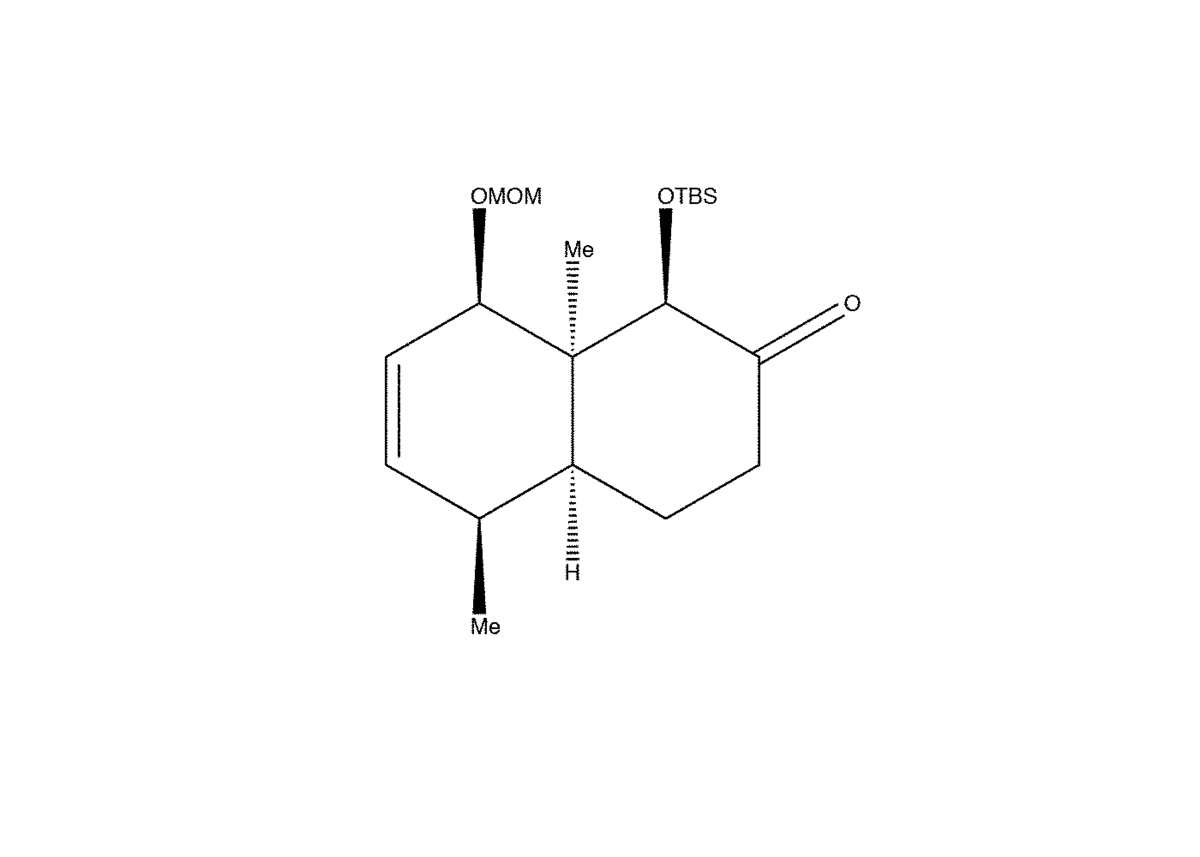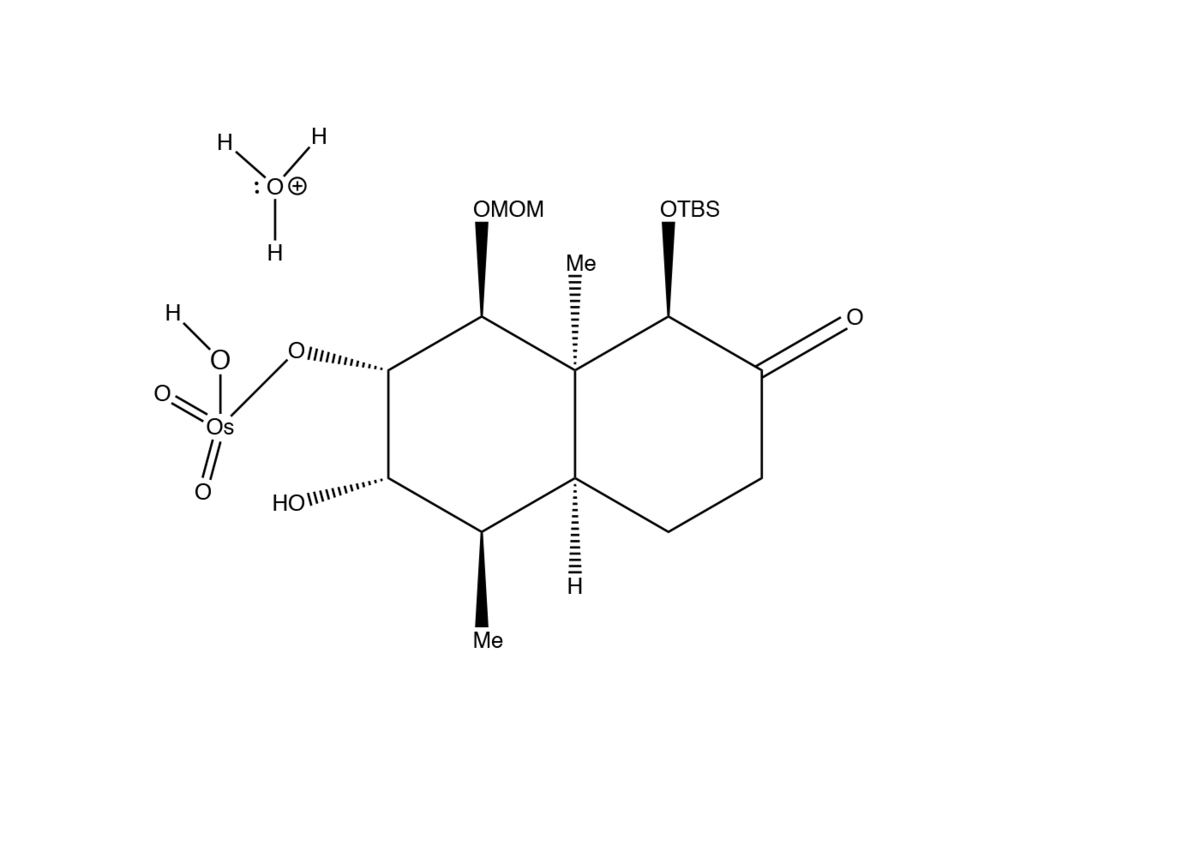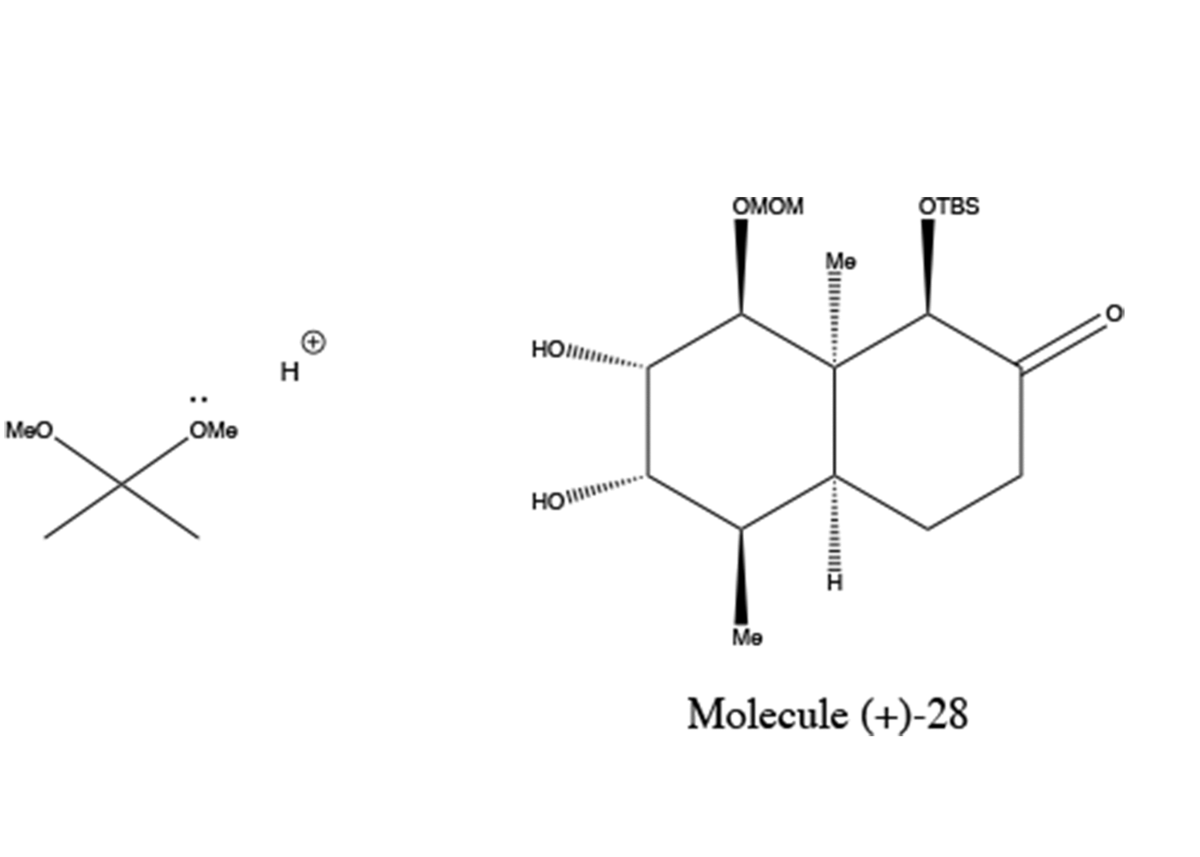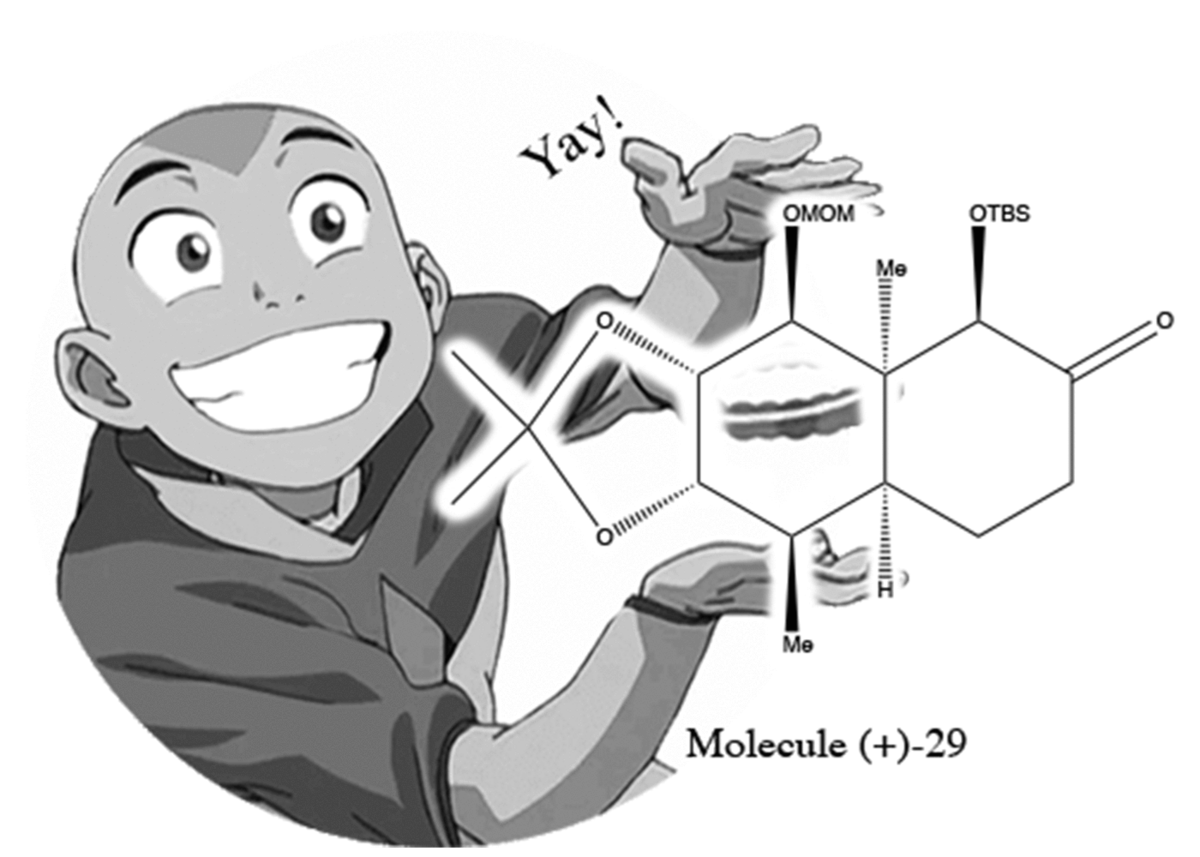
Dihydroxylation
1) Alkene double bond attacks OsO4 forming a Osmium Tetroxide complex.
2) Water attacks Osmium atom forming the first hydroxyl group.
3) Second water molecule attacks forming the final hydroxyl group.
---------------------------------------------------------------------------------------------------------------------------------------
Cyclic Acetal Formation
4) Methoxy group on 2,2-dimethoxypropane is protonated
5) Protonated Methoxy group leaves as electrons from other methoxy group form a carbonyl
6) Hydroxyl group from Molecule (+)-28 attacks carbonyl carbon.
7) Methoxy group is protonated, making it a good leaving group
8) Oxygen “pushes” off the protonated methoxy group, forming a carbonyl.
9) The second hydroxyl group on Molecule (+)-28 then attacks the newly-formed carbonyl carbon, forming an acetal. This molecule is Molecule (+)-29