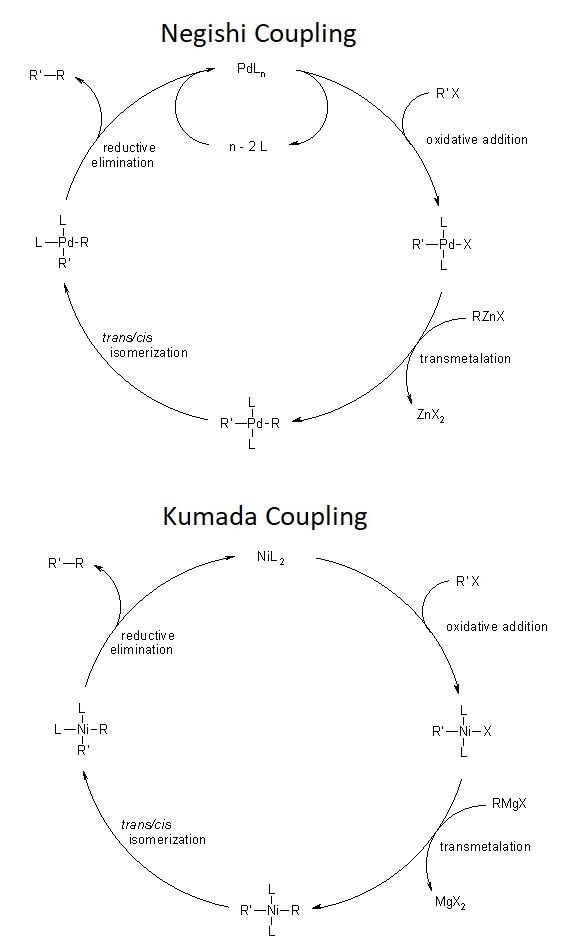
Negishi and Kumada cross-coupling reactions
What are they and why are they important?
Negishi, E.; Lious, S.Y.; Xu, C.; Huo, S. Org. Lett., 2002, 4, 261.
Previous studies by M. S. Kharasch and E. K. Fields in 1941 were the first investigations into the catalytic coupling of Grignard reagents with organic halides. Investigations involving cobalt, silver, copper, and iron gave complications and poor yield. Makoto Kumada first introduced the successful use of Ni catalysts in 1972, followed by the development of Pd as a transition metal catalyst by the Murahashi group in 1974. In 1976, El-ichi Negishi began studying the cross-coupling of organoaluminum reagents, with Ni and Pd as metal catalysts. During his study, he observed reactions involving a Ni catalyst expressed stereospecific decay, but ones involving Pd did not. Further studies by Negishi lead to the combination of reaction conditions we see today, and him and others were awarded with the Nobel prize in 2010 for their achievements.
The Kumada and Negishi cross-coupling techniques can be performed with a number of nickel or palladium catalysts. These catalytic structures are usually formed with phosphine ligands complexing to the transition metal catalyst in the form of ML₂X₂ where M is Pd or Ni and L is a phosphine ligand. The reaction conditions typically involve solvents such as tetrahydrofuran or diethyl ether, for their use in generating Grignard reagents. The application of both the Kumada and Negishi cross-coupling techniques are limited in large scale synthesis due to problematic functional group selectivity of Grignard reagents and the high sensitivity of aryl or alkyl zinc reagents with water and air. Use for the Kumada and Negishi cross-coupling techniques is typically employed in the total synthesis of natural products thanks to its increased reactivity and a more environmentally friendly zinc catalyst as opposed to tin and other metal coupling techniques.