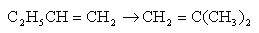Chapter 1: Mole Balances
Whenever the media player appears, audio is available for use, starting with the one below.Topics
- Chemical Identity
- Reaction Rate
- General Mole Balance Equation
- Mole Balance on Different Reactor Types
- Self Test Exercises
| Chemical Identity | TOP |
A chemical species is said to have reacted when it has lost its chemical identity. The identity of a chemical species is determined by the kind, number, and configuration of that species' atoms.
Three ways a chemical species can lose its chemical identity:
1. decomposition 2. combination 3. isomerization
| Reaction Rate | top |
The reaction rate is the rate at which a species looses its chemical identity per unit volume. The rate of a reaction can be expressed as the rate of disappearance of a reactant or as the rate of appearance of a product. Consider species A:
A → B
rA = the rate of formation of species A per unit volume
-rA = the rate of a disappearance of species A per unit volume
rB = the rate of formation of species B per unit volumeExample: A → B
If B is being created at a rate of 0.2 moles per decimeter cubed per second (i.e. the rate of formation of B is rB = 0.2 mole/dm3/s),
then A is disappearing at the same rate (-rA = 0.2 mole/dm3/s). This also means that the rate of formation of A is rA = -0.2 mole/dm3/s.
For a catalytic reaction, we refer to -rA', which is the rate of disappearance of species A on a per mass of catalyst basis.
NOTE: dCA/dt is not the rate of reactionConsider species j:
- rj is the rate of formation of species j per unit volume [e.g. mol/dm3*s]
- rj is a function of concentration, temperature, pressure, and the type of catalyst (if any)
- rj is independent of the type of reaction system (batch, plug flow, etc.)
- rj is an algebraic equation, not a differential equation
We use an algebraic equation to relate the rate of reaction, -rA, to the concentration of reacting species (e.g., CA) and to the temperature (T) at which the reaction occurs [e.g. -rA = k(T)CA2].
| General Mole Balance Equation | top |
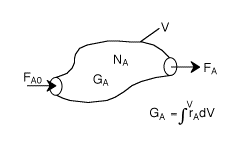
FA0= Entering molar flow rate of A (mol/time)
FA= Exiting molar flow rate of A (mol/time)
GA= Rate of generation(formation) of A (mol/time)
V = Volume (vol e.g. m3)
rA= rate of generation(formation) of A (mole/time•vol)
NA= number of moles of A inside the system Volume V (mol)
t = time (e.g. s)
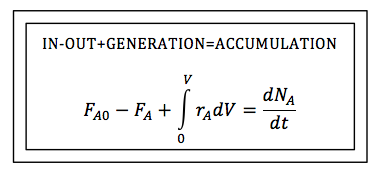
| Mole Balance on Different Reactor Types | top |
The General Mole Balance Equation (GMBE) applied to the four major reactor types (and the general reaction, A → B):
| Reactor | Differential | Algebraic | Integral | ||
|---|---|---|---|---|---|
| Batch |
|
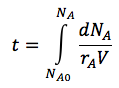 |
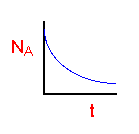 |
|
|
| CSTR |
|
|
|||
| PFR |
|
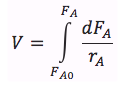 |
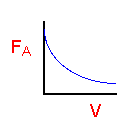 |
|
|
| PBR |
|
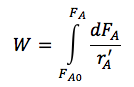 |
 |
|
| Self Test Exercises | top |
The following humorous video is set to Randy Newman's song "You've got a friend in me" was made by Professor Lane's 2008 Chemical Reaction Engineering class at the University of Alabama, Tuscaloosa.
The following animation is an excerpt taken from the lecture slides that Professor Fogler uses in his Reactions course, illustrating the importance of "Keeping Up"