Chapter 2: Conversion and Reactor Sizing
Example - Use Levenspiel plots to calculate conversion from known reactor volumes
Pure A is fed at a volumetric flow rate of 1000 dm3 /h and at a concentration of 0.005 mol/dm3 to an existing CSTR, which is connected in series to an existing tubular reactor.
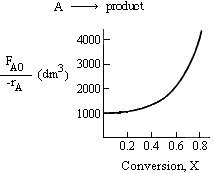
Solution
By trial and error, we find that a conversion of 0.6 gives the appropriate CSTR volume of 1200 dm3
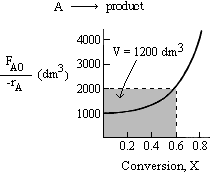
Therefore, the intermediate conversion is X = 0.6
Similarly for the PFR, through trial and error, we find that a conversion of 0.8 gives the appropriate PFR volume of 600 dm3
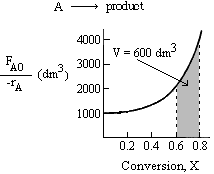
Therefore, the final conversion is X = 0.8