Chapter 2: Conversion and Reactor Sizing
Old Exam Questions
The adiabatic exothermic irreversible gas phase reaction
![]()
is to be carried out in a flow reactor for a stoichiometric feed of A and B.
Additional Information
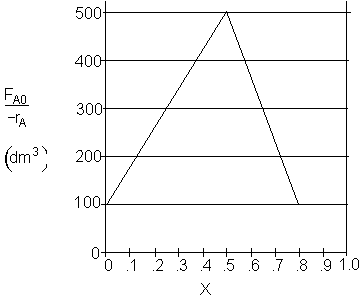
a) What PFR volume is necessary to achieve 50% conversion?
![]()
V1 =
$V_1 = (500 - 100) (0.5) = 200 dm^3$
$V_1 = (500 - 100) (0.5) + (100)(0.5) = 250 dm^3$
$V_1 = \dfrac{1}{2}(500 - 100) (0.5) + (100)(0.5) = 150 dm^3$
b) What CSTR volume is necessary to achieve 50% conversion?
V1 =
$V_1 = \dfrac{1}{2}(500) (0.5) = 125 dm^3$
$V_1 = (500) (0.5) = 250 dm^3$
$V_1 = (500 - 100) (0.5) = 200 dm^3$
c) What CSTR volume must be added to raise the conversion in Part (b) to 80%?
V2 =
$V_2 = (100) (0.8) = 80 dm^3$
$V_2 = (500 - 100) (0.8) = 160 dm^3$
$V_2 = (100) (0.8 - 0.5) = 30 dm^3$
d) What PFR volume must be added to raise the conversion in Part (b) to 80%?
V2 =
$V_2 = \dfrac{1}{2}(500 - 100) (0.8 - 0.5) = 60 dm^3$
$V_2 = \dfrac{1}{2}(500 - 100) (0.8 - 0.5) + 100(0.8 - 0.5)= 90 dm^3$
$V_2 = (500-100) (0.8 - 0.5)+100(0.8 - 0.5) = 150 dm^3$
Solution
a) What PFR volume is necessary to achieve 50% conversion? V1 = 150 dm3
![]()
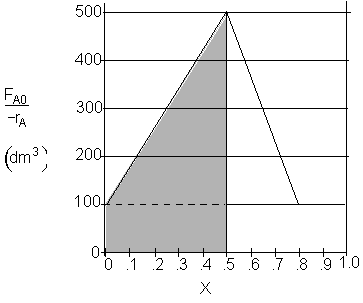
![]()
b) What CSTR volume is necessary to achieve 50% conversion? V1 = 250 dm3
![]()
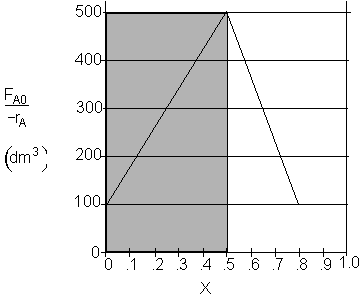
Shaded Area: ![]()
c) What CSTR volume must be added to raise the conversion in Part (b) to 80%? V2 = 30 dm3
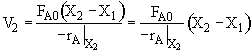
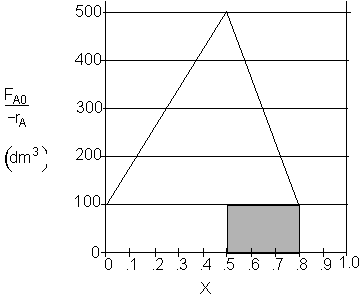
Shaded Area: ![]()
d) What PFR volume must be added to raise the conversion in Part (b) to 80%? V2 = 90 dm3
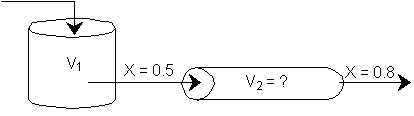
![]()
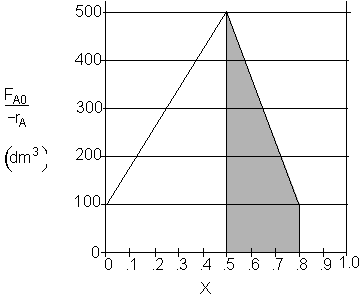
Shaded Area: ![]()