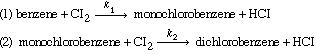Chapter 8: Multiple Reactions
Additional Homework Problems
CDP8-AB
| The chlorination of benzene is carried out in a CSTR under the following conditions: 55°C, liquid saturated with chlorine gas at a pressure of 1 atm, and a benzene conversion of 30%. The reaction rates are given by |
|
k 1 C b lb mol/
gal and the rate of monochlorobenzene disappearance by reaction (2) is k 2 C m lb mol/
gal |
||
|
where k 1
= 0.412 h-1, k2 = 0.055 h-1, and Cb
and Cm are the concentrations of benzene and monochlorobenzene
in lb mol/gal. Assume that the feed concentration of benzene (Cb0)
is 0.10 lb mol/ gal and that liquid density is independent of composition. Do not
assume that the formation of dichlorobenzene is negligible. What reactor volume is
required to produce 10 lb mol of monochlorobenzene per hour? (O. M. Fuller, McGill
University, Montreal) [1st Ed. P9-14] |