Chapter 8: Multiple Reactions
Frequently Asked Questions (FAQs)
- When do you need to consider overall selectivity (or yield)? What is
the advantage of having the two different kinds of selectivity (or yield)?
The instantaneous selective guides our initial reactor selection. For a CSTR the instantaneous selectivity is the same as overall selectivity.
- What is the purpose of the instantaneous selectivity parameter? It
seems to be just a descriptive term?
No, it helps to synthesize reaction schemes
- Why do you use a CSTR if you want to keep CA low and a
PFR when you want it high? This refers specifically to the home problem?
CSTRs operate at the lowest concentration. Feed drops to the low concentration immediately upon entry. PFR concentration starts high at entrance and drops slowly as one moves down the reactor.
- What happens when you have multiple reactions that are reversible?
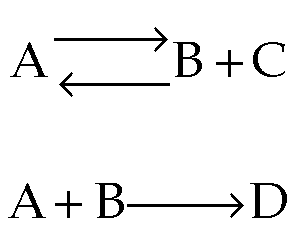
Just treat as:
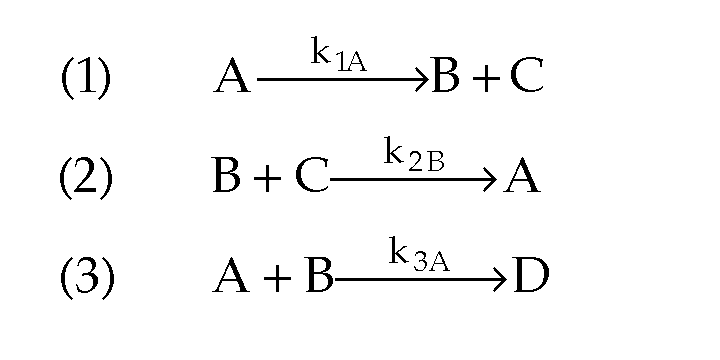
- When there are parallel reactions, the book discusses many cases
comparing the reaction orders of the equations. What kind of reactor should
be used if the two reactions have the same order (a1
= a2)?
In this case you need to work with temperature dependence to affect selectivity.
- In chapter 8, we consider the multiple reactions under isothermal
conditions, and we will cover in later chapters non-isothermal multiple
reactions. Is temperature the only main concern in the design for maximizing
selectivity?
We can vary inlet conditions and type of reactors
- Can catalysts be used in a competing reaction scenario? Can they
be used to give more of a preferred product?
Yes. Definitely!