Chapter 11: Nonisothermal Reactor Design: The Steady State Energy Balance and Adiabatic PFR Applications
Heats of Reaction
Calculate![]() ,
,![]() , and
, and![]() for the
reaction
for the
reaction ![]()
There are inerts I present in the system.
Additional information
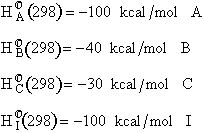
|
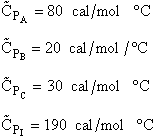
|
Hint 1: What is the heat of reaction at 298 C?
$(a) +10 kcal/mol A$
$(b) -10 kcal/mol A$
$(c) +30 kcal/mol A$
$(a) -10 \frac {cal}{mol A K}$
$(b) 10 \frac {cal}{mol A K}$
$(c) 90 \frac {cal}{mol A K}$
Calculate![]() ,
,![]() , and
, and![]() for
the reaction
for
the reaction
![]()
Additional information
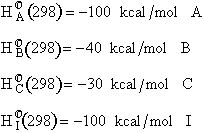
|
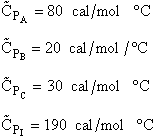
|
Solution
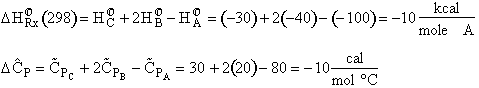
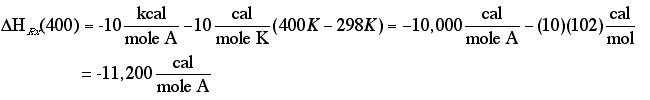
Note: The inerts NEVER enter into these calculations of