Chapter 12: Steady-State Nonisothermal Reactor Design: Flow Reactors with Heat Exchange
Adiabatic Reaction in a PBR
The elementary adiabatic reaction
![]() is carried out adiabatically in a PBR.
is carried out adiabatically in a PBR.

![]() We want to determine both the conversion and temperature profiles
We want to determine both the conversion and temperature profiles
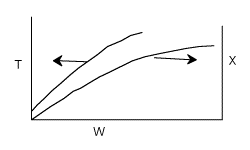 Write a Polymath program to plot X and T vs. W.
Write a Polymath program to plot X and T vs. W.
Solution
Mole Balance
![]() Rate Law
Rate Law
 Stoichiometry
Stoichiometry
 Energy Balance: Adiabatic
Energy Balance: Adiabatic
 Heat of reaction is independent of temperature.
Heat of reaction is independent of temperature.
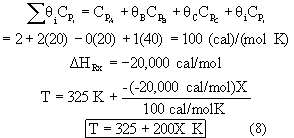

Why do you think the conversion and temperature curves become flat after a catalyst weight of 1200 kg?