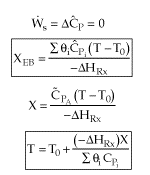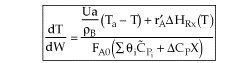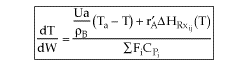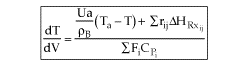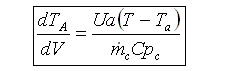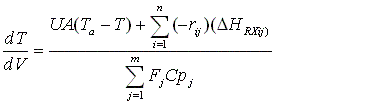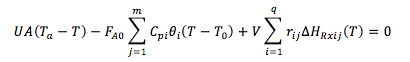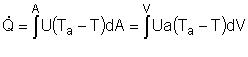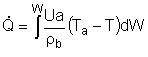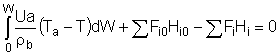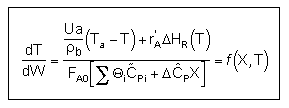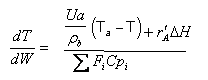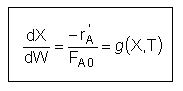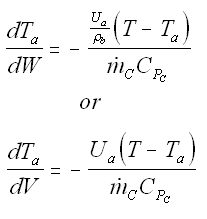Chapter 12: Steady-State Nonisothermal Reactor Design: Flow Reactors with Heat Exchange
Topics
- Overview of User Friendly Energy Balance Equations
- Evaluating the Heat Exchanger Term
- Multiple Steady States
- Multiple Reactions with Heat Effects
- Applications of the PFR/PBR User Friendly Energy Balance Equations
| User Friendly Energy Balance Equations | top |
The user friendly forms of the energy balance we will focus on are outlined in the following table.
User friendly equations relating X and T, and Fi and T 1. Adiabatic CSTR, PFR, Batch, PBR achieve this:
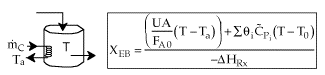
2. CSTR with heat exchanger, UA(Ta-T) and large coolant flow rate.
3A. In terms of conversion, X
3B. In terms of molar flow rates, Fi
4. For Multiple Reactions
5. Coolant Balance
|
||||||||||||||
| These equations are derived in the text. These are the equations that we will use to solve reaction engineering problems with heat effects. |
| Evaluating the Heat Exchanger Term | top |

|
|
Assuming the temperature inside the CSTR, T,
is spatially uniform: |
|
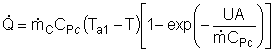
|
|
At high coolant flow rates the exponential term will be small, so we can expand the exponential term as a Taylor Series, where the terms of second order or greater are neglected, then: |
|
|
|
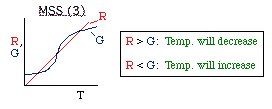
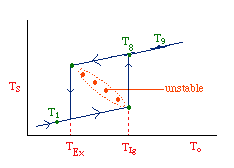
| Multiple Steady States (MSS) | top |
| From page 593 we can obtain | |
 |
|
where |
|
 |
|
 |
Now we need to find X. We do this by combining the mole balance, rate law, Arrhenius Equation, and stoichiometry.
For the first-order, irreversible reaction A --> B, we have:
|
||
where |
||
At steady state: |
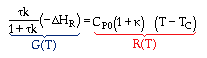 |
|
Substituting for k... |
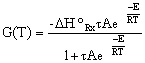
|
|
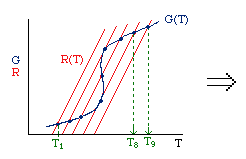 |
 |
|
| Multiple Reactions with Heat Effects | top |
To account for heat effects in multiple reactions, we simply replace the term (-delta HRX) (-rA) in equations (12-35) PFR/PBR and (12-40) CSTR by:
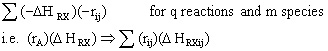
PFR/PBR
CSTR
These equations are coupled with the mole balances and rate law equations discussed in Chapter 6.
Complex Reactions
Example: Consider the following gas phase reactions
|
|
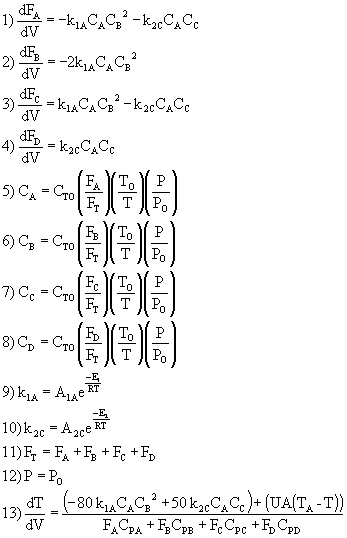
We now substitute the various parameter values (e.g. delta HRX, E, U) into equations (1)-(13) and solve simultaneously using Polymath.
| Applications of the PFR/PBR User Friendly Energy Balance Equations | top |
NOTE: The PFR and PBR formulas are very similar.
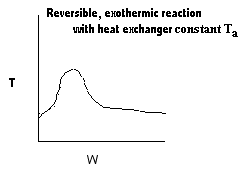 |
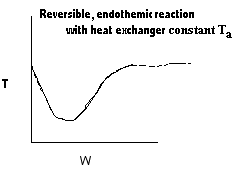 |
|
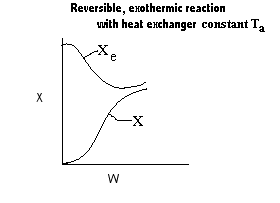 |
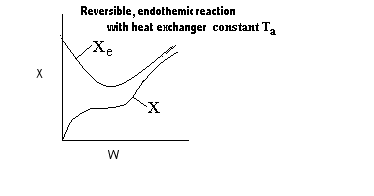 |
|
If we include pressure drop: |
||
C. $$ \frac{dp}{dW} = -\frac{\alpha}{2p}\frac{F_{T}}{F_{T0}}\frac{T}{T_{0}} = \frac{-\alpha(1+\epsilon X)}{2p}(\frac{T}{T_{0}}) = h(X,T) $$ |
||
Note: the pressure drop will be greater for exothermic adiabatic reactions than it will be for isothermal reactions |
||
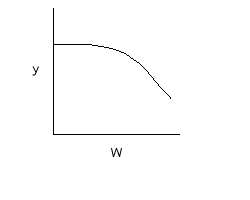 |
||
Balance on Heat Exchanger Coolant Solve simultaneously using an ODE solver (Polymath/MatLab). If Ta is not constant, then we must add an additional energy balance on the coolant fluid: |
||
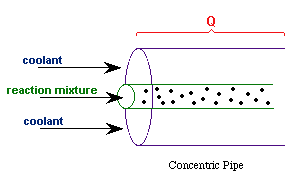
| Co-Current Flow | 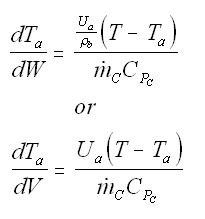 | |
| Counter-Current Flow |
with Ta = Tao at W = 0 |
|
For an exothermic reaction: with counter current heat exchange
|
|
A Trial and Error procedure for counter current flow problems is required to find exit conversion and temperature.
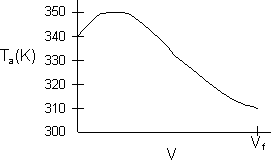
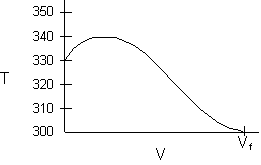
- Consider an exothermic reaction where the coolant stream enters at the end of the reactor at a temperature Ta0, say 300 K.
- Assume a coolant temperature at the entrance (X = 0, V = 0) to the reactor Ta2 =340 K.
- Calculate X, T, and Ta as a function of V. We can see that our guess of 340 K for Ta2 at the feed entrance (X = 0) gives a coolant temperature of 310 K, which does not match the actual entering coolant temperature of 300 K.
- Now guess a coolant temperature at V = 0 and X = 0 of 330 K. We see that the exit coolant temperature of Ta2 = 330 K will give a coolant temperature at V = V1 of 300 K.