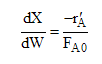Chapter 4: Stoichiometry
Rate Law in terms of Partial Pressures
An example of a typical rate law found in the catalystic engineering literature for the gas phase catalyzed reaction.
If the reaction follows an Eley-Rideal mechanism (Ch 10) it will take the form
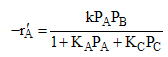
where k is the specific reaction rate 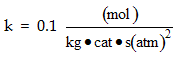 and KA and KC are the equilibrium adsorption constant of KA = 2 atm-1 and KC = 0.5 atm-1.
and KA and KC are the equilibrium adsorption constant of KA = 2 atm-1 and KC = 0.5 atm-1.
(a) Express as a function of conversion for an entering partial pressure of 8 atm Using the ideal gas law we know
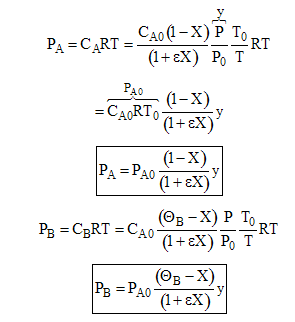
similarly
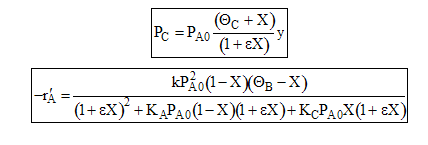
For an equal molar feed
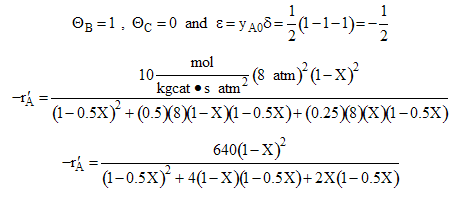
We now can solve for the catalyst weight to achieve a specific conversion by combining -r`A in terms of X with the mole balance