Chapter 4: Stoichiometry
Old Exam Questions
The elementary gas phase reaction
![]()
takes place isothermally in a flow reactor with no pressure drop. The feed is equal molar in A and B. Write the rate of reaction, -rA, solely as a function of the conversion, X [i.e. -rA = f(X)].

(a) $-r_{A} = k_{A}C_{A}{C_{A}}^{\frac{1}{2}}$
(b) $-r_{A} = k_{A}C_{A}{C_{B}}$
(c) $-r_{A} = k_{A}{C_{A}}^2{C_{B}}$
Hint 3: What is CA as a function of X?
(a) $C_{A} = C_{A0}(1 - x)$
(b) $C_{A} = C_{A0}\frac{(1-x)}{(1+3x)}$
(c) $C_{A} = \frac{C_{A0}(1-x)}{1+\frac{3}{2}x}$
Hint 4: What is CB as a function of X?
(a) $C_{B} = C_{A0}(\frac{1}{2} - \frac{1}{2}x)$
(b) $C_{B} = C_{A0}(1 - \frac{1}{2}x)$
(c) $C_{B} = C_{A0} \frac{1-\frac{1}{2}x}{1+\frac{3}{2}x}$
Hint 1:
Remember that the rate law for gases includes volume change.
![]()
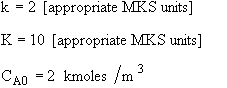
![]()
![]()
Hint 2
![]()
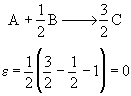
Hint 3
What is CA as a function of X?
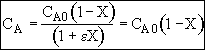
Hint 4
What is CB as a function of X?
From the stoichiometry: b/a = 1/2
Equal Molar:
![]()
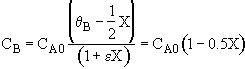
Solution
We see species A is the limiting reactant as the feed is equal molar in A and B, but one mole of B consumes two moles of A.
![]()
![]()
Need to put everything in terms of per mole of limiting reactant A.
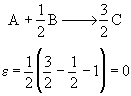
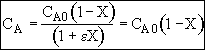
From the stoichiometry: b/a = 1/2
Equal Molar:
![]()
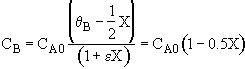
![]()
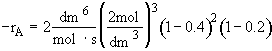
![]()