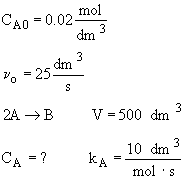Chapter 6: Isothermal Reactor Design: Molar Flow Rates
Liquid Phase CSTR
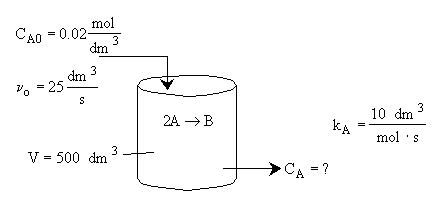
Hint 1: What are the mole balances for A and B?
$ (a) V = \frac{F_{A0} - F_{A}}{-r_{A}} \textrm{,} V = \frac{F_{B}}{r_{B}}$
$ (b) V = \frac{F_{A0} - F_{A}}{r_{B}} \textrm{,} V = \frac{F_{B}}{-r_{B}}$
$ (c) V = \frac{\upsilon_{0}(C_{A0} - C_{A})}{-r_{A}} \textrm{,} V = \frac{\upsilon_{0}C_{B}}{-r_{A}}$
Hint 3: What is the combined rate law and mole balance?
$ (a) V = \frac{\upsilon_{0}(C_{A0}-C_{A})}{k{C_{A}}^2}$
$ (b) V = \frac{\upsilon_{0}C_{B}}{k{C_{A}}^2} $
Hint 1
Mole Balances
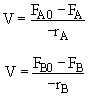
Hint 2
Rate Law
![]()
Stoichiometry Liquid n = no
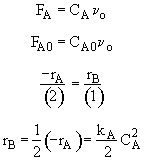
Hint 3
Combine
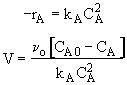
Mole Balances
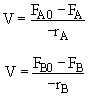
Rate Law
![]()
Stoichiometry Liquid n = no
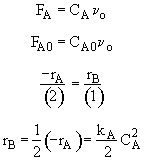
Combine
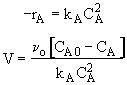
Dividing by vo,
and letting t =
![]() , the
, the
![]()
Rearranging
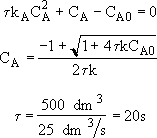
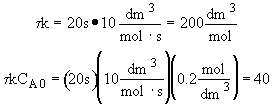
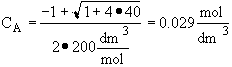
Back calculate X
![]()
![]()