| t (min) | 0 | 10 | 20 | 30 |
| CA(mol/dm3) | 1 | 0.6 | 0.4 | 0.3 |
The reactionSolutionis carried out in a constant volume batch reactor. Determine the reaction order and specific reaction rate from the following data.
t (min) 0 10 20 30 CA(mol/dm3) 1 0.6 0.4 0.3
t CA 0 1 0.4 -(0.6-1.0)/(10-0)=0.04 10 0.6 0.2 -(0.4-0.6)/(20-10)=0.02 20 0.4 0.1 -(0.2-0.4)/(30-20)=0.01 30 0.3 First find
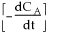
t CA 0 1 0.05 0.04 10 0.6 0.03 0.02 20 0.4 0.015 0.01 30 0.3 0.005 Now plot
versus t and it should be a straight line.
The plot is essentially linear, therefore the reaction is zero order. From the slope of the line we find
k=0.00167 mol/dm3 min.What two things are wrong with this solution?
|
|
1) The graphical differentiation of the data is incorrect asis plotted as function of
. First, even the data of
versus
is not plotted correctly. According to this incorrect analysis, it should rise linearly. However, this fact is irrelevant because the x-axis should be time, not
. The correct plot is as follows.
t CA 0 1 0.053 10 0.6 0.028 20 0.4 0.017 30 0.3 0.014 2) The plot to determine the reaction order and reate constant is incorrect. Combined mole balance and postulated rate law is
taking the natural log of both sides
Plot
versus ln CA or plot
versus CA on log-log paper to determine the reaction order. The correct plot on log-log is as follows.
The reaction order is
therefore,
When CA=1.0 mol/dm3 then