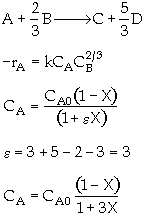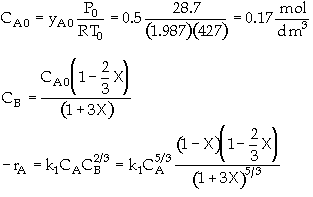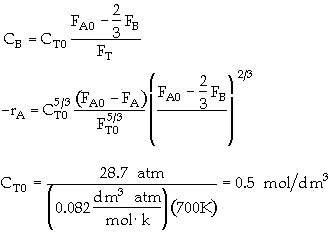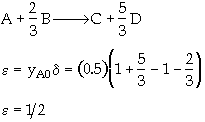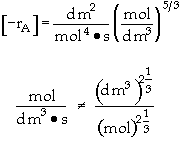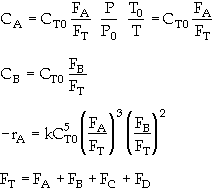The elementary gas phase reaction
![]()
is carried out in a flow reactor operated isothermally at 427ĮC and 28.7 atmospheres. Pressure drop can be neglected. Express the rate law and the concentration of each species as a function of conversion and as a function of the total molar flow rates. The entering volumetric flow rate is 10 dm3/s and the specific reaction rate is 200 dm12/mol4*s. The feed is equal molar in A and B.