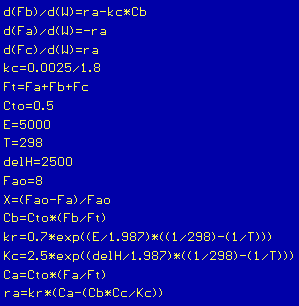
The equations used are the same as those found in the Type 4 Home Problem solution. The only difference is that we now make kR and Kc temperature dependent. The equations and knowns can be plugged into POLYM
ATH and the numbers varied.
Varying kc - graphs
Changing the value of kc changes the rate at which B passes through the membrane. When it is small, little B passes through the membrane so the reaction is not driven to the right. The flowrate of B stays roughly the same as C.
When kc is large, B can pass easily through the membrane, driving the reaction to the right. The flow rate of B takes a large dive as the concentration of B becomes high enough for B to start passing rapidly through the membrane.
Varying Temperature - graphs
Changing the temperature affects the reaction rate constant and the equilibrium constant. At low temperatures the reaction progresses very slowly and little product is formed. When the temperature is high the reaction progresses very quickly and the rea
ction is essentially over at a short distance into the reactor.
Varying Flowrate - graphs
Changing the flowrate changes the rate at which the materials travel through the reactor. When the flowrate is small, the reactants travel slowly through the reactor and have plenty of time to react. As the flow rate increases the materials pass quickly
through the reactor and do not have as much time for the reaction to progress.
Other than the variables we have already changed, the problem could also ask to vary groups such as kc and KC or ratios such as kR/kc.
 Back to problem statement
Back to problem statement
CRE >
Thoughts >
Ten Types >
Heterogeneous Example 7



![]() Back to problem statement
Back to problem statement