For this module there are a couple of topics that need to be reviewed or
dicussed:
1) Semibatch reactor equations
2) Modifications due to reactive distillation
![]()
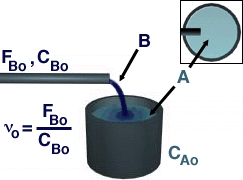
Shown above is a representation of a typical semibatch reactor. B is
fed to A, which is already in the reactor, and there are no outputs
(evporation included). To model a semibatch reactor, start with a mole
balance on each species :
Starting with species A :
|
|
|
|
C and D are similar :
| |
|
||||
Species B is fed to the reactor
making its mole balance equation slightly different :
| |
We now have all the equations needed to describe the changes in concentration of the different species in the reactor over time. However, since there is a feed stream, the volume in the reactor will change. So, we need to develop an equation describing how the volume in the reactor changes with time.
Starting with a mass balance and remebering that mass can neither be
created nor destroyed :
The mass terms in the equation can be replaced by :
giving :
Assuming that the system is at constant density leads to :
That is the last equation needed to model the semibatch reactor.
![]()
| Mole Balance | Mass Balance | |||
| Rate Law | ||||
Now we can move on and talk about reactive distillation.
|
|