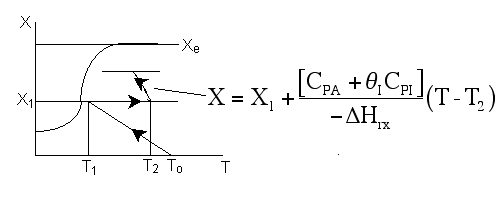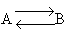
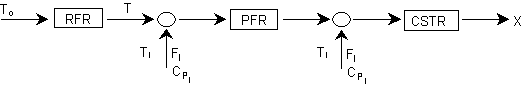
The following endothermic reaction is to be carried out in an interstage reactor system in which inerts are injected between the reactors to increase the temperature.
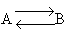
An inert I is injected at the points shown below:
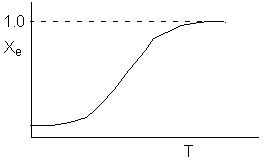
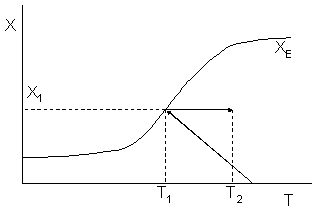
 Sketch the conversion-temperature trajectory for the above reaction system.
Sketch the conversion-temperature trajectory for the above reaction system.
Hint 2: Sketch X vs. T using the energy balance equation
Hint 3: Complete an energy balance around injector point to calculate T2
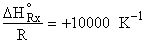 and Keq=0.1 and T2
and Keq=0.1 and T2
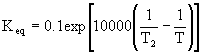
The adiabatic energy balance is
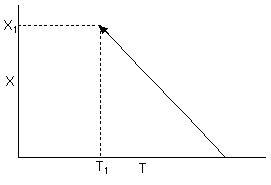
![]() From the energy balance we know the temperature decreases with increasing conversion.
(The endothermic heat of reaction is positive.)
From the energy balance we know the temperature decreases with increasing conversion.
(The endothermic heat of reaction is positive.)
Only A enters:
![]()
or
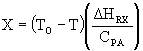
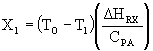
where (DHRX/CPA) is the slope in each case.
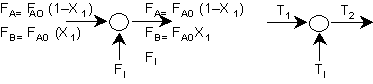 Energy Balance around junction:
Energy Balance around junction:
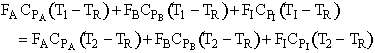 Solving for T2
Solving for T2
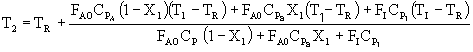
If CPA = CPB,
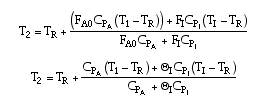
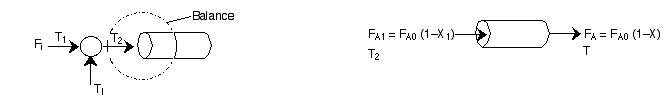
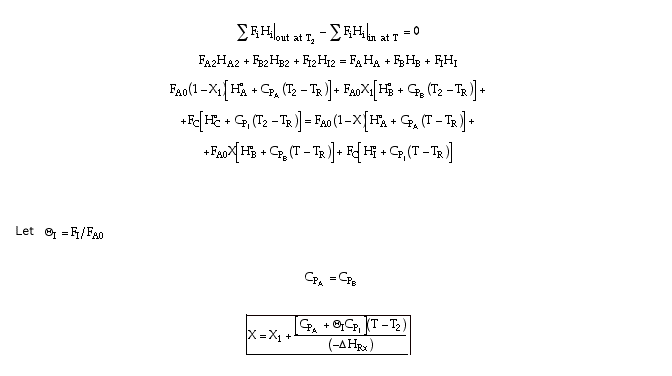
Because we are only adding inerts, we can still treat X as the overall conversion up to any point.
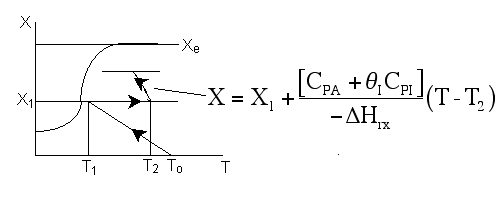
An inert I is injected at the points shown below:
 Sketch the conversion-temperature trajectory for an endothermic reaction.
Sketch the conversion-temperature trajectory for an endothermic reaction.
Solution
For an endothermic reaction, the equilibrium conversion increases with increasing T. For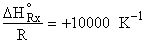 and Keq=0.1 and T2
and Keq=0.1 and T2
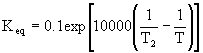

The adiabatic energy balance is
![]() From the energy balance we know the temperature decreases with increasing conversion.
(The endothermic heat of reaction is positive.)
From the energy balance we know the temperature decreases with increasing conversion.
(The endothermic heat of reaction is positive.)
Only A enters:
![]()
or
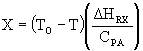
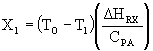
where (DHRX/CPA) is the slope in each case.

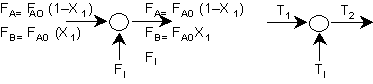 Energy Balance around junction:
Energy Balance around junction:
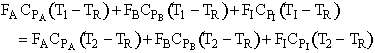 Solving for T2
Solving for T2
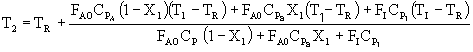

If CPA = CPB,
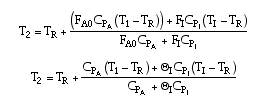
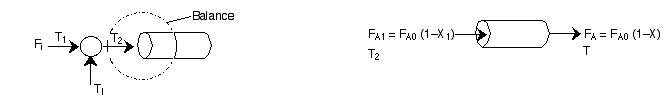
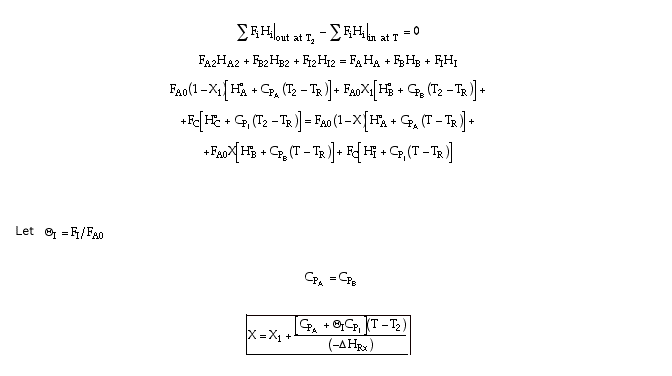
Because we are only adding inerts, we can still treat X as the overall conversion up to any point.