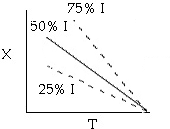
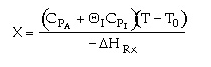
1. The following conversion temperature relationship is for a
adiabatic reaction, A -> B, containing 50% inerts:
Sketch X vs. T for when a) the inerts are increased to 75% and b) when
the inerts are decreased to 25%.
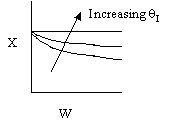
2. The following conversion temperature
relationship is also for an adiabatic reaction, A -> B, containing
50% inerts:
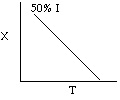
Sketch X vs. T for when a) the inerts are increased to 75% and b) when
the inerts are decreased to 25%.
Solution
|
|
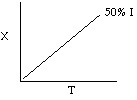
The following conversion temperature relationship is for a adiabatic reaction, A -> B, containing 50% inerts:

Sketch X vs. T for when a) the inerts are increased to 75% and b) when
the inerts are decreased to 25%.
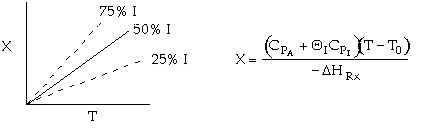
As inerts are increased, you reach the same conversion at a lower temperature.
The following conversion temperature relationship is also for an
adiabatic reaction, A -> B, containing 50% inerts:

Sketch X vs. T for when a) the inerts are increased to 75% and b) when
the inerts are decreased to 25%.
 
|
|
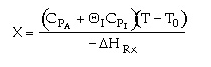
|
As you increase inerts, there is more sensible heat to supply the
reaction and the temperature does not drop as much when the inerts are
increased.
Back to Problem 2