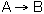
1. The rate law
2. The rate of formation of species A is per unit volume is(a.) is a differential equation T F
(b.) relates reaction rate and concentration of reacting species T F
3.(c.) the rate of generation of species A per unit volume T F
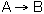
At a particular time t, the rate of formation of B in the reaction, rB, is 10 mole/dm3*min. Which of the following are true?
The rate of disappearance of B is -10 moles/dm3*min.
The rate of formation of A is -10 mole/dm3*min.
The rate of disappearance of A is 10 moles/dm3*min.
rA = -10 moles/dm3*min
-rA = 10 moles/dm3*min
-rB = -10 moles/dm3*min
Some of the above
All of the above
None of the above
A will disappear faster if a magician is present
1 (b.) TRUE
-rA = function (temperature and reacting species concentration), e.g.
IfDCP = 0, then otherwise,
see p. 930 of text.
1 (c.) FALSE It's also valid in Canada and the northern Provinces
Return to Problem 1
1 (d.) TRUE (Page 5 of Text)
Return to Problem 1
2 (a.) TRUE (Page 8 of Text)
rA=moles of A formed, per unit time, per unit volume
[rA]~[mol/dm3 s]
2 (b.) FALSE (Pages 3 and 5 of Text)
-rA is the rate of disappearance of A, [mol/dm3 s]
2 (c.) TRUE
rate of formation=rate of generation
2 (d.) FALSE (Page 6 of Text)
r'A is the rate of disappearance of A per mass of catalyst, i.e. grams of catalyst [mol/kg cat s]
2 (e.) FALSE (a & c are true)
Return to Problem 2
2 (f.) TRUE
The Toronto Maple Leafs Hockey Team has little use for rate of formation of species A.
Solutions
1 (a.) FALSE
F. The rate law is an algebraic equation relating the rate of reaction with the concentration of the reacting species. Example, for the elementary reaction
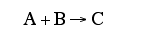
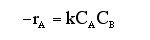
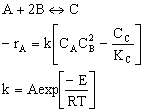
![]()
Solution
h. (all are true)
Explanation
Consider a constant volume (V = V0) batch system
Equivalently 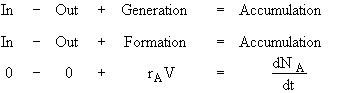
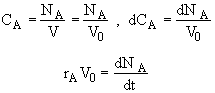
![]()
![]()
 :
:![]()
At t = 0 then![]() . A moment later at t = 0.01 sec then
. A moment later at t = 0.01 sec then![]() . If
. If![]() were to be positive, the
concentration of A would increase with time. However, A is being consumed so the
concentration of A is decreasing with time. For small times we use a different
formula to find
were to be positive, the
concentration of A would increase with time. However, A is being consumed so the
concentration of A is decreasing with time. For small times we use a different
formula to find![]() .
.
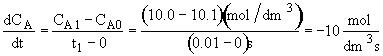
The rate of formation of A is
![]()
![]()
![]()
“B” is being formed
![]()