- decomposition
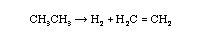
- combination
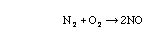
- isomerization
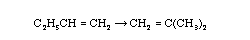
| Chemical Identity | top |
A chemical species is said to have reacted when it has lost its chemical identity. The identity of a chemical species is determined by the kind, number, and configuration of that species' atoms.
Reaction Video: Carbon Dioxide and Magnesium
Three ways a chemical species can lose its chemical identity:
- decomposition
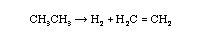
- combination
- isomerization
| Reaction Rate (p.2) | top |
The reaction rate is the rate at which a species looses its chemical identity per unit volume. The rate of a reaction can be expressed as the rate of disappearance of a reactant or as the rate of appearance of a product. Consider species A:
AB
rA = the rate of formation of species A per unit volume
-rA = the rate of a disappearance of species A per unit volume
rB = the rate of formation of species B per unit volumeExample: A
B
If B is being created at a rate of 0.2 moles per decimeter cubed per second, ie, the rate of formation of B is,
rB = 0.2 mole/dm3/sThen A is disappearing at the same rate:
-rA = 0.2 mole/dm3/s
the rate of formation of A is
rA = -0.2 mole/dm3/s
For a catalytic reaction, we refer to -rA', which is the rate of disappearance of species A on a per mass of catalyst basis.
NOTE: dCA/dt is not the rate of reactionConsider species j:
We use an algebraic equation to relate the rate of reaction, -rA, to the concentration of reacting species (e.g., CA) and to the temperature (T) at which the reaction occurs [e.g. -rA = k(T)CA2].
Reaction Video: Sodium with Chlorine
| General Mole Balance Equation (p.6) | top |
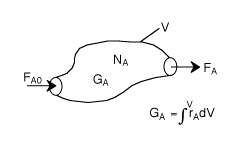
FA0= Entering molar flow rate of A (mol/time)
FA= Exiting molar flow rate of A (mol/time)
GA= Rate of generation(formation) of A (mol/time)
V = Volume (vol e.g. m3)
rA= rate of generation(formation) of A (mole/time•vol)
NA= number of moles of A inside the system Volume V (mols)
Mole Balance on Different Reactor Types (p.25) top The GMBE applied to the four major reactor types (and the general reaction, A->B):
Reactor Differential Algebraic Integral Batch CSTR PFR PBR
Self Test Exercises top
The following movie was made by the students of Professor Alan Lane's chemical reaction engineering class at the University of Alabama Tuscaloosa
* All chapter references are for the 4th Edition of the text Elements of Chemical Reaction Engineering .