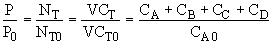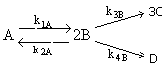
Problem
Consider the elementary gas phase reactions
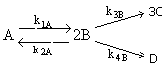
Set up the equations to calculate the concentration of each species as a function of time in a constant volume batch reactor. The reaction is carried out isothermally.
Solution
A combined mole balance(1) and rate laws(2)
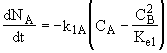
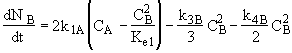
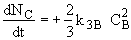
(3) Stoichiometry
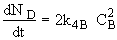
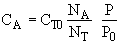
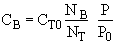
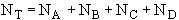
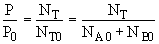
at t = 0 then NA = NA0 and
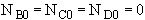
These equations will be typed into Polymath.
What five things are wrong with each of the first three steps of the multiple reaction algorithm?
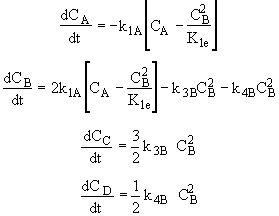
(1)Mole Balance: V missing on right hand side of each of the combined mole balance and rate law equations (1) through (4).
(2)Stoiciometry: The gas reaction occurs in a constant volume rigid container, V = V0. Therefore the equations [5) and (6)] for the concentration of the reacting species are not correct
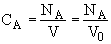
(3)Rate laws, relative rates, and net rates: The net rate of reaction is not correct for B,C, and D, i.e., equations (2), (3), and (4).
Since V = V0
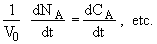
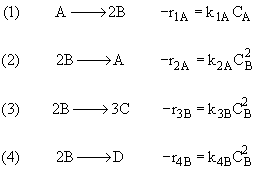
(1)
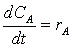
(2)
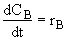
(3)
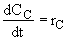
(4)
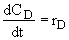
Net Rates
Species A
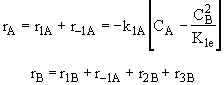
Species B
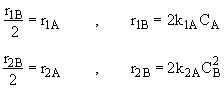
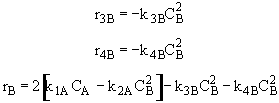
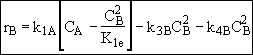
Species C
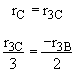
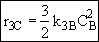
Species D
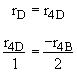
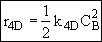
Stoichiometry
The pressure inside the vessel is